20. October 2022
Transglutaminase antibodies and neurological manifestations of gluten sensitivity
by Daniel Aeschlimann and Marios Hadjivassiliou
Sensitivity to gluten can present with neurological problems, with cerebellar ataxia being the most frequent manifestation and referred to as gluten ataxia (GA). GA is the commonest immune mediated ataxia. Up to 60% of patients with GA have no evidence of enteropathy (coeliac disease-CD) on small bowel biopsy, yet such patients respond to a gluten free diet (Hadjivassiliou et al., 2013). Originally, the diagnosis of gluten ataxia was considered in those patients serologically positive for antibodies to gliadins (antigliadin antibodies, AGA), proteins derived from wheat gluten. In patients with sensitivity to gluten, systemic levels of antigliadin IgA/IgG appear to mirror the immune reaction triggered by gluten, including their reduction in response to clinical improvement following gluten free diet. However, these serological assays have relatively low specificity, and availability of AGA testing is now limited due to the development of more specific second generation anti-deamidated gliadin peptide and anti-TG2 IgA assays for CD diagnosis. Furthermore, the fact that the positive cut-off in most AGA assays is based on testing patients with CD and hence applicable to CD diagnosis only, means that only physicians/neurologists that are familiar with the limitations associated with this may be able to use such assays for the diagnosis of GA.
Sensitivity to gluten results in a systemic immune-mediated disease that can present with diverse manifestations (gut, skin, brain). Besides the strong gluten-specific T cell response, another hallmark of gluten-related diseases (GRD) is a robust autoantibody response to one or more transglutaminase (TG) isozymes. Formation of TG-gluten peptide complexes allows B cells carrying TG-specific IgD to take up and subsequently present gluten-peptides complexed to MHC, and thereby allowing for B cell activation and differentiation in the absence of TG-specific T cells. It has been shown that stable thioester complexes of gluten peptides with the active site Cys residue formed during catalysis play a role in this process and such complexes are indeed formed by all TGs to which autoantibodies are developed in GRD (Stamnaes et al., 2010).
In patients with neurological deficits, there is a bias of the immune response towards TG6 (Hadjivassiliou et al., 2008). Most of the available evidence pertains to cerebellar ataxia but it is likely that a spectrum of neurological conditions including ataxia with myoclonus, peripheral neuropathy, gluten encephalopathy (headache with white matter abnormalities, sometimes associated with vascular dementia) may be caused by pathogenic antibodies (Hadjivassiliou et al., 2010). Evidence for a link between neurological deficits and GRD is three-fold. Firstly, the production of anti-TG6 antibodies is gluten dependent which substantiates the link to the gluten-specific T cell population (Hadjivassiliou et al., 2013). Secondly, alterations in specific brain regions are manifest in CD patients with circulating anti-TG6 antibodies, providing evidence for a link between brain atrophy and autoimmunity to TG6 (Hadjivassiliou et al., 2019). And thirdly, similar to anti-TG3 autoantibodies and dermatitis herpetiformis, passive transfer of the disease through antibodies in an animal model directly implicates these in pathogenesis (Boscolo et al., 2010). TG6 expression is indeed associated with neurogenesis in the central nervous system, and a role of the enzyme in neurons controlling motor function is strongly suggested by an association of mutations in the TGM6 gene with spinocerebellar ataxia 35 (Thomas et al., 2013; Tripathy et al., 2017). Molecular modeling and biochemical assays, have shown that TG6 is allosterically regulated by Ca2+ and GTP similar to TG2, and that disease associated mutations compromise regulation of enzymatic function (Thomas et al., 2013). Both, compromised enzyme folding and functionality appear to have an impact on neuronal survival, albeit through distinct molecular mechanisms (Tripathy et al., 2017).
Detection of autoantibodies to TG6 may not only facilitate diagnosis of GRD in a neurology setting, but screening of patients with established celiac disease for circulatory antibodies to TG6 may provide an opportunity to identify patients at risk of developing neurological problems. There is growing evidence that circulatory antibodies to TG6 (as well as TG3) are a prelude to extraintestinal disease, often detectable long before any manifestations become symptomatic (Hadjivassiliou et al., 2020).
This research overview reflects the professional opinion of the authors and although invited, was not sponsored or incentivised by the company.
References:
Boscolo et al., PLOS ONE 2010, 5:e9698 (1-9); Hadjivassiliou et al., Ann. Neurol. 2008. 64, 332-43; Hadjivassiliou et al., Lancet Neurol. 2010, 9:318-30; Hadjivassiliou et al., Neurology 2013, 80:1-6; Stamnaes et al., Amino Acids 2010, 39:1183-91; Hadjivassiliou et al., 2019. Clin. Gastroenterol. Hepatol. 17: 2678-2686; Thomas et al., Amino Acids 2013, 44:167-77. Tripathy et al., 2017. Hum. Mol. Genet. 26: 3749-3762; Hadjivassilou et al., 2020. Nutrients 12: 2884.
The authors:


Sensitivity to gluten can present with neurological problems, with cerebellar ataxia being the most frequent manifestation and referred to as gluten ataxia (GA). GA is the commonest immune mediated ataxia. Up to 60% of patients with GA have no evidence of enteropathy (coeliac disease-CD) on small bowel biopsy, yet such patients respond to a gluten free diet (Hadjivassiliou et al., 2013). Originally, the diagnosis of gluten ataxia was considered in those patients serologically positive for antibodies to gliadins (antigliadin antibodies, AGA), proteins derived from wheat gluten. In patients with sensitivity to gluten, systemic levels of antigliadin IgA/IgG appear to mirror the immune reaction triggered by gluten, including their reduction in response to clinical improvement following gluten free diet. However, these serological assays have relatively low specificity, and availability of AGA testing is now limited due to the development of more specific second generation anti-deamidated gliadin peptide and anti-TG2 IgA assays for CD diagnosis. Furthermore, the fact that the positive cut-off in most AGA assays is based on testing patients with CD and hence applicable to CD diagnosis only, means that only physicians/neurologists that are familiar with the limitations associated with this may be able to use such assays for the diagnosis of GA.
Sensitivity to gluten results in a systemic immune-mediated disease that can present with diverse manifestations (gut, skin, brain). Besides the strong gluten-specific T cell response, another hallmark of gluten-related diseases (GRD) is a robust autoantibody response to one or more transglutaminase (TG) isozymes. Formation of TG-gluten peptide complexes allows B cells carrying TG-specific IgD to take up and subsequently present gluten-peptides complexed to MHC, and thereby allowing for B cell activation and differentiation in the absence of TG-specific T cells. It has been shown that stable thioester complexes of gluten peptides with the active site Cys residue formed during catalysis play a role in this process and such complexes are indeed formed by all TGs to which autoantibodies are developed in GRD (Stamnaes et al., 2010).

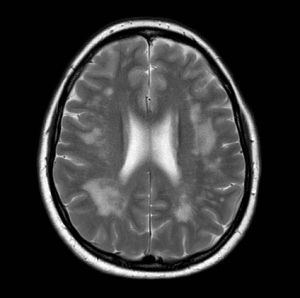
MR brain imaging of a patient with gluten encephalopathy showing excessive white matter signal change. Such patients usually present with intractable headaches. Gluten free diet results in elimination of the headaches and stops the progression of the white matter changes. If untreated such patients may end up with vascular dementia.
Courtesy of Marios Hadjivassiliou.
Courtesy of Marios Hadjivassiliou.
In patients with neurological deficits, there is a bias of the immune response towards TG6 (Hadjivassiliou et al., 2008). Most of the available evidence pertains to cerebellar ataxia but it is likely that a spectrum of neurological conditions including ataxia with myoclonus, peripheral neuropathy, gluten encephalopathy (headache with white matter abnormalities, sometimes associated with vascular dementia) may be caused by pathogenic antibodies (Hadjivassiliou et al., 2010). Evidence for a link between neurological deficits and GRD is three-fold. Firstly, the production of anti-TG6 antibodies is gluten dependent which substantiates the link to the gluten-specific T cell population (Hadjivassiliou et al., 2013). Secondly, alterations in specific brain regions are manifest in CD patients with circulating anti-TG6 antibodies, providing evidence for a link between brain atrophy and autoimmunity to TG6 (Hadjivassiliou et al., 2019). And thirdly, similar to anti-TG3 autoantibodies and dermatitis herpetiformis, passive transfer of the disease through antibodies in an animal model directly implicates these in pathogenesis (Boscolo et al., 2010). TG6 expression is indeed associated with neurogenesis in the central nervous system, and a role of the enzyme in neurons controlling motor function is strongly suggested by an association of mutations in the TGM6 gene with spinocerebellar ataxia 35 (Thomas et al., 2013; Tripathy et al., 2017). Molecular modeling and biochemical assays, have shown that TG6 is allosterically regulated by Ca2+ and GTP similar to TG2, and that disease associated mutations compromise regulation of enzymatic function (Thomas et al., 2013). Both, compromised enzyme folding and functionality appear to have an impact on neuronal survival, albeit through distinct molecular mechanisms (Tripathy et al., 2017).
Detection of autoantibodies to TG6 may not only facilitate diagnosis of GRD in a neurology setting, but screening of patients with established celiac disease for circulatory antibodies to TG6 may provide an opportunity to identify patients at risk of developing neurological problems. There is growing evidence that circulatory antibodies to TG6 (as well as TG3) are a prelude to extraintestinal disease, often detectable long before any manifestations become symptomatic (Hadjivassiliou et al., 2020).
This research overview reflects the professional opinion of the authors and although invited, was not sponsored or incentivised by the company.
References:
Boscolo et al., PLOS ONE 2010, 5:e9698 (1-9); Hadjivassiliou et al., Ann. Neurol. 2008. 64, 332-43; Hadjivassiliou et al., Lancet Neurol. 2010, 9:318-30; Hadjivassiliou et al., Neurology 2013, 80:1-6; Stamnaes et al., Amino Acids 2010, 39:1183-91; Hadjivassiliou et al., 2019. Clin. Gastroenterol. Hepatol. 17: 2678-2686; Thomas et al., Amino Acids 2013, 44:167-77. Tripathy et al., 2017. Hum. Mol. Genet. 26: 3749-3762; Hadjivassilou et al., 2020. Nutrients 12: 2884.
The authors:

Daniel Aeschlimann, PhD is Professor of Biological Sciences at College of Biomedical and Life Sciences, Cardiff University, UK.
His research focuses on understanding and manipulating the interface between extracellular matrix and the diversity of cells in the craniofacial complex with the long-term goal of counteracting pathological processes leading to tissue destruction or create functional tissue through application of life science principles.
His research focuses on understanding and manipulating the interface between extracellular matrix and the diversity of cells in the craniofacial complex with the long-term goal of counteracting pathological processes leading to tissue destruction or create functional tissue through application of life science principles.

Professor Marios Hadjivassiliou is a Consultant Neurologist at the Academic Department of Neurosciences, Sheffield Teaching Hospitals NHS Trust and University of Sheffield, UK.
He has conducted and published extensive research into Gluten Ataxia and other neurological manifestations of gluten sensitivity, having first described the condition in the 1990’s. He runs a dedicated weekly clinic for patients with neurological manifestations of sensitivity to gluten.
He has conducted and published extensive research into Gluten Ataxia and other neurological manifestations of gluten sensitivity, having first described the condition in the 1990’s. He runs a dedicated weekly clinic for patients with neurological manifestations of sensitivity to gluten.
 Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering  Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor  Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation