
Zedira is a privately held, clinical-stage biopharmaceutical company specialized in transglutaminase.
Zedira’s drug candidate ZED12271 was the first transglutaminase blocker ever tested in clinical trials.
The peptidomimetic compound showed proof-of-concept in a phase 2a study in celiac disease patients. In addition, ZED1227 has a placebo-like safety profile.
For the first time, a transglutaminase is clinically validated as a druggable target.
Zedira is conducting several drug discovery programs targeting dysregulated human transglutaminases. The company established a pipeline of small-molecule drug candidates adapted to specific indications. Please refer to the Medicine section for comprehensive information.
Our pipeline is available on request (contact@zedira.com).

Martin Hils and Ralf Pasternack,
managing partners of Zedira, founded the company in 2007.
“Transglutaminase is our inspiration – improving health and creating value is our mission.“
Specialists for Transglutaminases
Zedira is the global brand for specialty reagents for research & development as well as diagnostics in the transglutaminase field. More than 300 unique and high-quality products help accelerate transglutaminase-related research – both in academia and industry.
Scientists from academia and industry (including several of the top 15 pharmaceutical companies) benefit from Zedira’s cutting-edge products and customized services.
Zedira’s recombinant, ultra-pure, and highly active microbial transglutaminase (MTG) is a unique tool to generate homogenous antibody drug conjugates (ADCs). Today, ADCs manufactured with Zedira’s Andracon™ are entering clinical trials. Find detailed information about Andracon™ in our MTG-Handbook.
Zedira products are made in Germany by Zedira’s highly skilled professionals in an ISO9001:2015 certified facility. The excellent feedback from our customers motivates us daily to provide our high-quality transglutaminase specialty reagents to researchers world wide.
Diagnostics
Zedira’s ISO9001:2015 certified facility enables the production of high-quality diagnostic antigens in the celiac field, including TG2, TG3, TG6, and DGPs (deamidated gliadin peptides). Moreover, Zedira’s proprietary anti-TG6-ELISA allows the detection of autoantibodies to neuronal transglutaminase (TG6), a novel marker in the field of gluten-related neurological disorders.
1 Büchold C, Hils M, Gerlach U, Weber J, Pelzer C, Heil A, Aeschlimann D, Pasternack R. Features of ZED1227: The First-In-Class Tissue Transglutaminase Inhibitor Undergoing Clinical Evaluation for the Treatment of Celiac Disease. Cells. 2022; 11(10):1667. https://doi.org/10.3390/cells11101667
 Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering 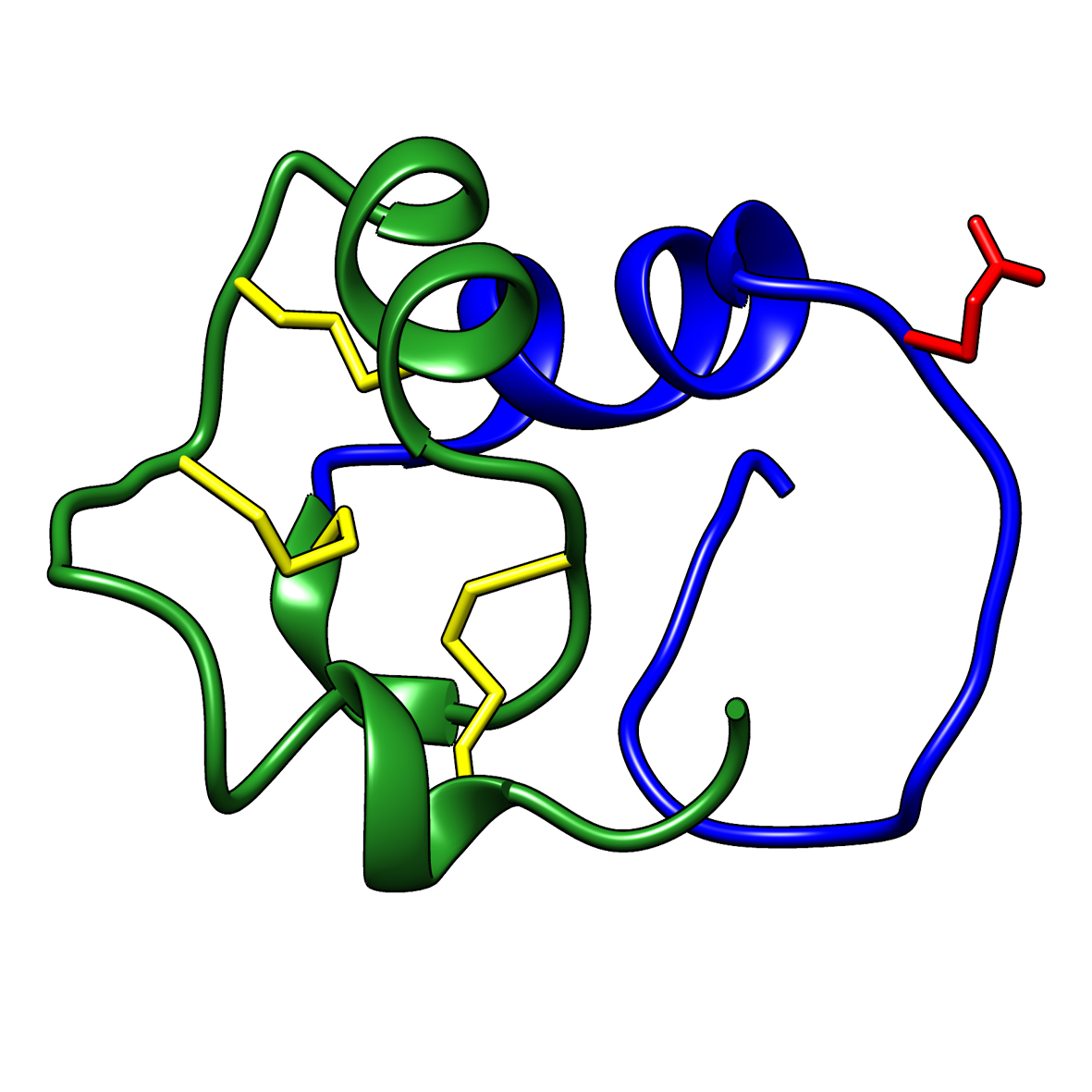 Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor 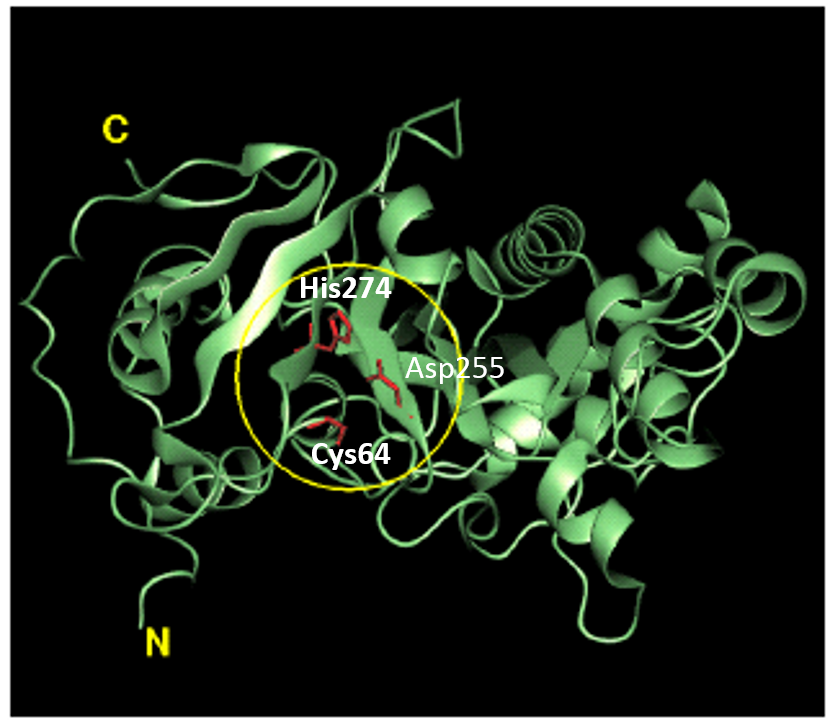 Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation? 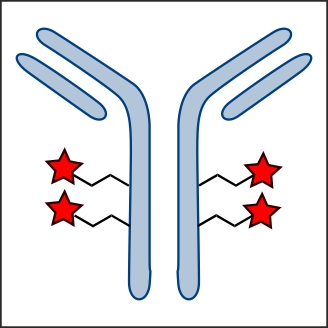 Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation