Back to all products
Antibodies and Microarrays
Antibodies are important tools in many research and diagnostic applications.
Zedira offers antibodies – polyclonal as well as monoclonal – raised against:
Antibody epitope mapping
PEPperPRINT and Zedira proudly present the new PEPperCHIP® Transglutaminase Microarray!
Standard and Custom PEPperCHIP® Peptide Microarrays are synthesized with a unique laser printer-based method directly on-chip and are used for antibody epitope mapping, immunological research, and biomarker discovery.
Read more about PEPperCHIP® Laser Printing Platform
Antibodies labeled with fluorescent dyes
Zedira provides many of its polyclonal antibodies with a fluorescent label like FITC or phycoerythrin or as a biotinylated version. Of course, antibodies with other tags are available on demand.
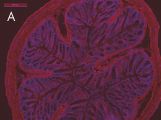
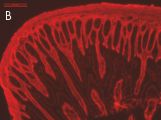
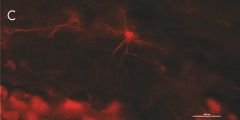
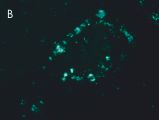
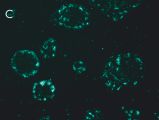
Indirect immunostaining
(A) Anti-TG2 (A014): mouse colon section (1:100);
(B) Anti-TG2 (A014): mouse small intestinal mucosa;
(C) Anti-TG6 (A017): astrocyte in mouse corpus callosum.
(A/B) J. Knauer, Fraunhofer Gesellschaft Leipzig; (C) A. Schulze-Krebs, S. v. Hörsten, University Erlangen
In many applications, the need for a fluorescently labeled antibody exists, ranging from fluorescence microscopy to fluorescence-activated cell sorting (FACS).
Direct immunostaining
(A) Transmitted light microscopy, magnification 1:40;
(B) FITC-Anti-TG2 (A028): fluorescence microscopy, magnification 1:40;
(C) FITC-Anti-TG2 (A028): fluorescence microscopy, magnification 1:20.
(A-C) W. Dieterich, University Erlangen-Nürnberg
Change Notification for Monoclonal Antibodies:
The antibody formulation has changed in 2021 to a formulation containing 50% glycerol (Change notification antibody formulation.pdf)
Zedira offers antibodies – polyclonal as well as monoclonal – raised against:
- All human transglutaminases
- Guinea pig liver transglutaminase
- Bacterial transglutaminase
- The transglutaminase reaction product: Nε-(γ-glutamyl)-L-lysine-isopeptide bond
- Fibrinolytic enzymes: Plasmin and its zymogen (plasminogen)
- Gliadin-variants
Antibody epitope mapping
PEPperPRINT and Zedira proudly present the new PEPperCHIP® Transglutaminase Microarray!
Standard and Custom PEPperCHIP® Peptide Microarrays are synthesized with a unique laser printer-based method directly on-chip and are used for antibody epitope mapping, immunological research, and biomarker discovery.
Read more about PEPperCHIP® Laser Printing Platform
Antibodies labeled with fluorescent dyes
Zedira provides many of its polyclonal antibodies with a fluorescent label like FITC or phycoerythrin or as a biotinylated version. Of course, antibodies with other tags are available on demand.



Indirect immunostaining
(A) Anti-TG2 (A014): mouse colon section (1:100);
(B) Anti-TG2 (A014): mouse small intestinal mucosa;
(C) Anti-TG6 (A017): astrocyte in mouse corpus callosum.
(A/B) J. Knauer, Fraunhofer Gesellschaft Leipzig; (C) A. Schulze-Krebs, S. v. Hörsten, University Erlangen
In many applications, the need for a fluorescently labeled antibody exists, ranging from fluorescence microscopy to fluorescence-activated cell sorting (FACS).



Direct immunostaining
(A) Transmitted light microscopy, magnification 1:40;
(B) FITC-Anti-TG2 (A028): fluorescence microscopy, magnification 1:40;
(C) FITC-Anti-TG2 (A028): fluorescence microscopy, magnification 1:20.
(A-C) W. Dieterich, University Erlangen-Nürnberg
Change Notification for Monoclonal Antibodies:
The antibody formulation has changed in 2021 to a formulation containing 50% glycerol (Change notification antibody formulation.pdf)
 Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering  Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor  Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation