
We measure activity in your transglutaminase samples!
Background:
Transglutaminase (Microbial transglutaminase, MTG) is a processing aid widely used to improve meat, fish, bakery, and dairy products. Transglutaminase cross-linking can modulate the physical and textural properties of various protein-containing foods.
Transglutaminase Activity:
At Zedira, we determine the activity of your transglutaminase sample on a fee-for-service basis.
How to get your sample measured at Zedira?
- Sample shipment
Send us at least 50 g of your sample using a carrier of your choice.
Ship it to: Zedira GmbH, Roesslerstrasse 83, 64293 Darmstadt, Germany.
Please inform us via e-mail about your shipment (contact@zedira.com) and provide the expected activity range.
Samples typically are MTG concentrates (2,000 – 3,000 U/g) or ready-to-use MTG formulations (60 – 200 U/g).
- Sample measurement
The transglutaminase activity of your samples is measured using the standard hydroxamate assay (see assay principle below).
Two independent measurements, in triplicate, are performed per sample.
Our internal quality standards require inter-assay and intra-assay deviations of no more than 5%.
- Results
The transglutaminase activity of each sample is stated in a Certificate of Analysis (example for download).
The certificate of analysis is sent to you via e-mail within 1-2 working days after receipt of your sample.
- Fee for service
The assay fee per sample is 275 €.
When sending 3 samples at the same time, the fee per analysis is reduced to 247.5 €.
The invoice is sent by e-mail and can be paid by bank transfer or credit card.
Phone: +49 6151 66628-0
E-mail: contact@zedira.com
The transglutaminase activity assay principle:
The Standard Hydroxamate Assay uses Z-Gln-Gly-OH as the peptidic glutamine substrate and hydroxylamine as the amine donor.
In the presence of MTG, hydroxylamine is enzymatically incorporated into the peptide to form Z-glutamyl-hydroxamate-glycine. The hydroxamate forms a red colored complex with iron (III) ions, which is quantified at 525 nm.
One unit of microbial transglutaminase activity is defined as the amount of enzyme, that causes the formation of 1.0 μmole of hydroxamate per minute at 37°C (Folk and Cole, 1966).
(FAQ - Determination of MTG activity.pdf)
 Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible? 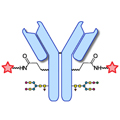 Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering 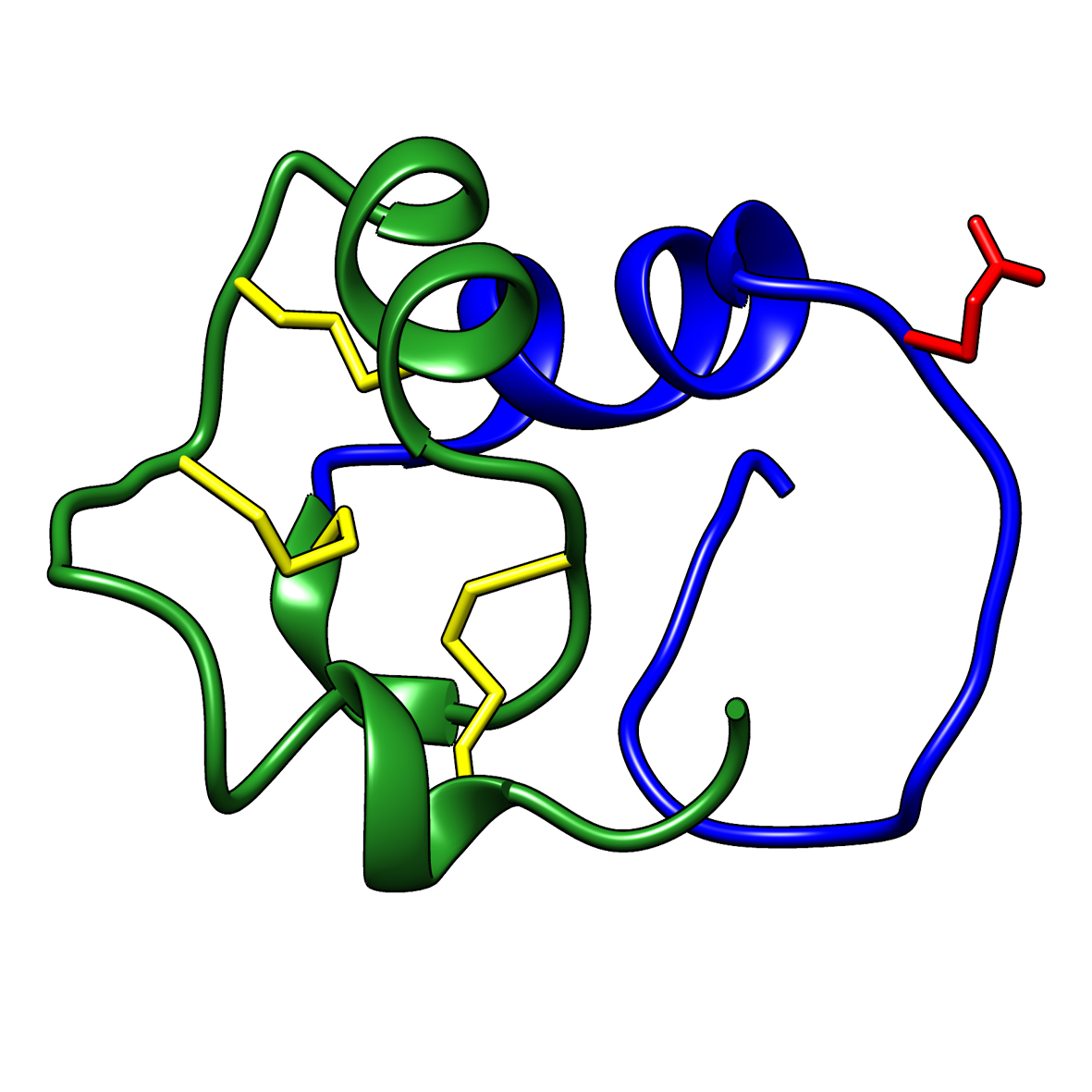 Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor 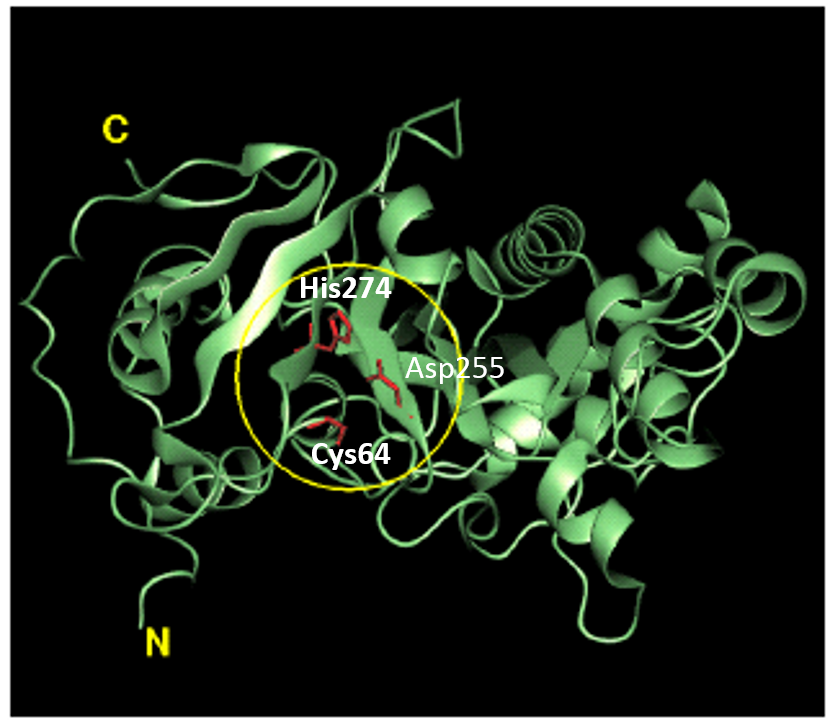 Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation? 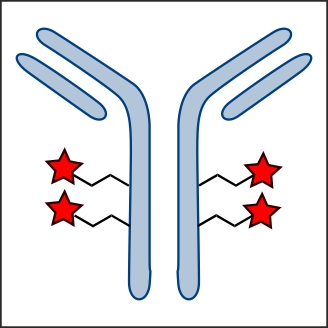 Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation