Back to all products
| Isopeptidase-Fluorogenic Assays |
| Transglutaminase fluorogenic Activity Assay |
| Photometric Activity Assay |
| Enzyme Immuno Assay |
| Glutamine-donor peptides |
| Amine-donors |
| Protein Substrates |
| „Hitomi“-peptides |
Assays and Substrates
Transglutaminases are defined as protein-glutamine: amine glutamyl transferases (EC 2.3.2.13). They use a modified double-displacement mechanism to carry out an acyl transfer reaction between the γ-carboxamide group of a peptide-bound glutamine residue and the ε-amino group of a peptide-bound lysine. The active site consists of a catalytic triad (Cys, His, and Asp).
The active site cysteine reacts with the γ-carboxamide of the glutamine, forming a γ-glutamyl thioester releasing ammonia. This activated species subsequently reacts with nucleophilic primary amines, yielding either an isopeptide bond (pathway 1) or a (γ-glutamyl)amine bond (pathway 2). When an amine is not available, the acyl-enzyme intermediate reacts with water to yield glutamic acid (pathway 3).
Reaction pathway of transglutaminase
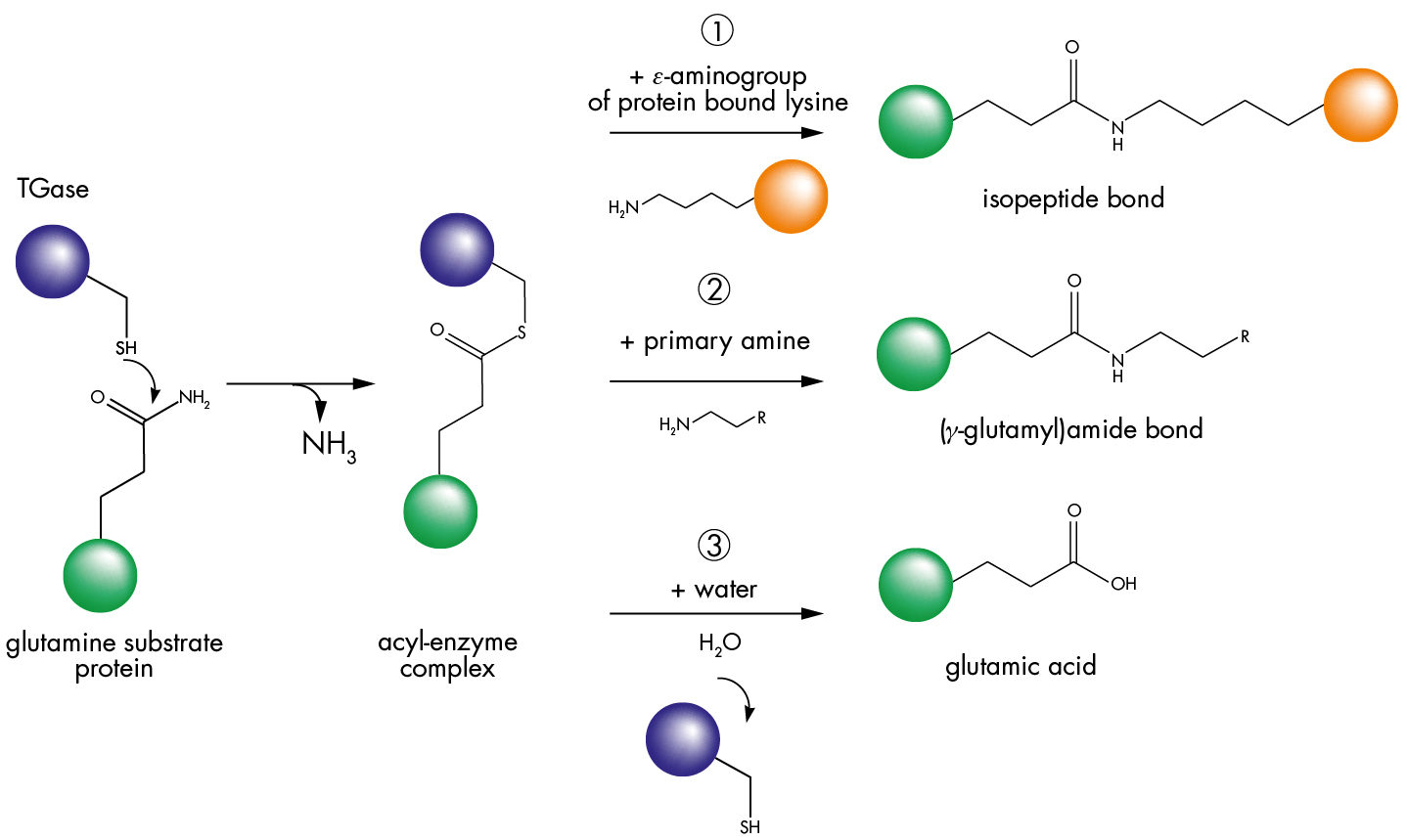
The incorporation of dansylcadaverine into casein (compareable to pathway 2) leads to an increase in fluorescence intensity. The principle is used in kit T036.
During the transpeptidation reaction, ammonia (NH3) is released. The amount of ammonia can be monitored using glutamate dehydrogenase and NADPH as a co-factor. Depending on the transglutaminase, either casein or synthetic peptides serve as acyl donor substrates. The reaction can be monitored online using a UV photometer at 340 nm. An activity assay using this principle is available for MTG (M001).
The kit M003 is the most sensitive allowing detection of TG2 in the picogram range using the incorporation of biotinylated peptides to microtiter plates displaying primary amines on the surface.
TG2 - Enzyme Immuno Assay (EIA):
The kit E018 is dedicated to determining the overall quantity of TG2. The principle behind this kit is a sandwich EIA based on two monoclonal antibodies against tissue transglutaminase (TG2). The kit provides coated plates and all the reagents necessary including a tissue transglutaminase calibrator.
The chromogenic assay kit Z009 uses Z-Gln-Gly as the amine acceptor substrate and hydroxylamine as an amine donor. In the presence of transglutaminase, hydroxylamine is incorporated forming glutamyl-hydroxamate, which develops a colored complex with iron (III) detectable at 525 nm (red).
The reverse reaction of TGs is measured in the isopeptidase assay platform including F001 (FXIII), F014 and F016 (TG2).
The active site cysteine reacts with the γ-carboxamide of the glutamine, forming a γ-glutamyl thioester releasing ammonia. This activated species subsequently reacts with nucleophilic primary amines, yielding either an isopeptide bond (pathway 1) or a (γ-glutamyl)amine bond (pathway 2). When an amine is not available, the acyl-enzyme intermediate reacts with water to yield glutamic acid (pathway 3).
Reaction pathway of transglutaminase

The incorporation of dansylcadaverine into casein (compareable to pathway 2) leads to an increase in fluorescence intensity. The principle is used in kit T036.
During the transpeptidation reaction, ammonia (NH3) is released. The amount of ammonia can be monitored using glutamate dehydrogenase and NADPH as a co-factor. Depending on the transglutaminase, either casein or synthetic peptides serve as acyl donor substrates. The reaction can be monitored online using a UV photometer at 340 nm. An activity assay using this principle is available for MTG (M001).
The kit M003 is the most sensitive allowing detection of TG2 in the picogram range using the incorporation of biotinylated peptides to microtiter plates displaying primary amines on the surface.
TG2 - Enzyme Immuno Assay (EIA):
The kit E018 is dedicated to determining the overall quantity of TG2. The principle behind this kit is a sandwich EIA based on two monoclonal antibodies against tissue transglutaminase (TG2). The kit provides coated plates and all the reagents necessary including a tissue transglutaminase calibrator.
The chromogenic assay kit Z009 uses Z-Gln-Gly as the amine acceptor substrate and hydroxylamine as an amine donor. In the presence of transglutaminase, hydroxylamine is incorporated forming glutamyl-hydroxamate, which develops a colored complex with iron (III) detectable at 525 nm (red).
The reverse reaction of TGs is measured in the isopeptidase assay platform including F001 (FXIII), F014 and F016 (TG2).
 Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering  Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor  Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation