Back to all products
| Isopeptidase-Fluorogenic Assays |
| Transglutaminase fluorogenic Activity Assay |
| Photometric Activity Assay |
| Enzyme Immuno Assay |
| Glutamine-donor peptides |
| Amine-donors |
| Protein Substrates |
| „Hitomi“-peptides |
Photometric Activity Assay
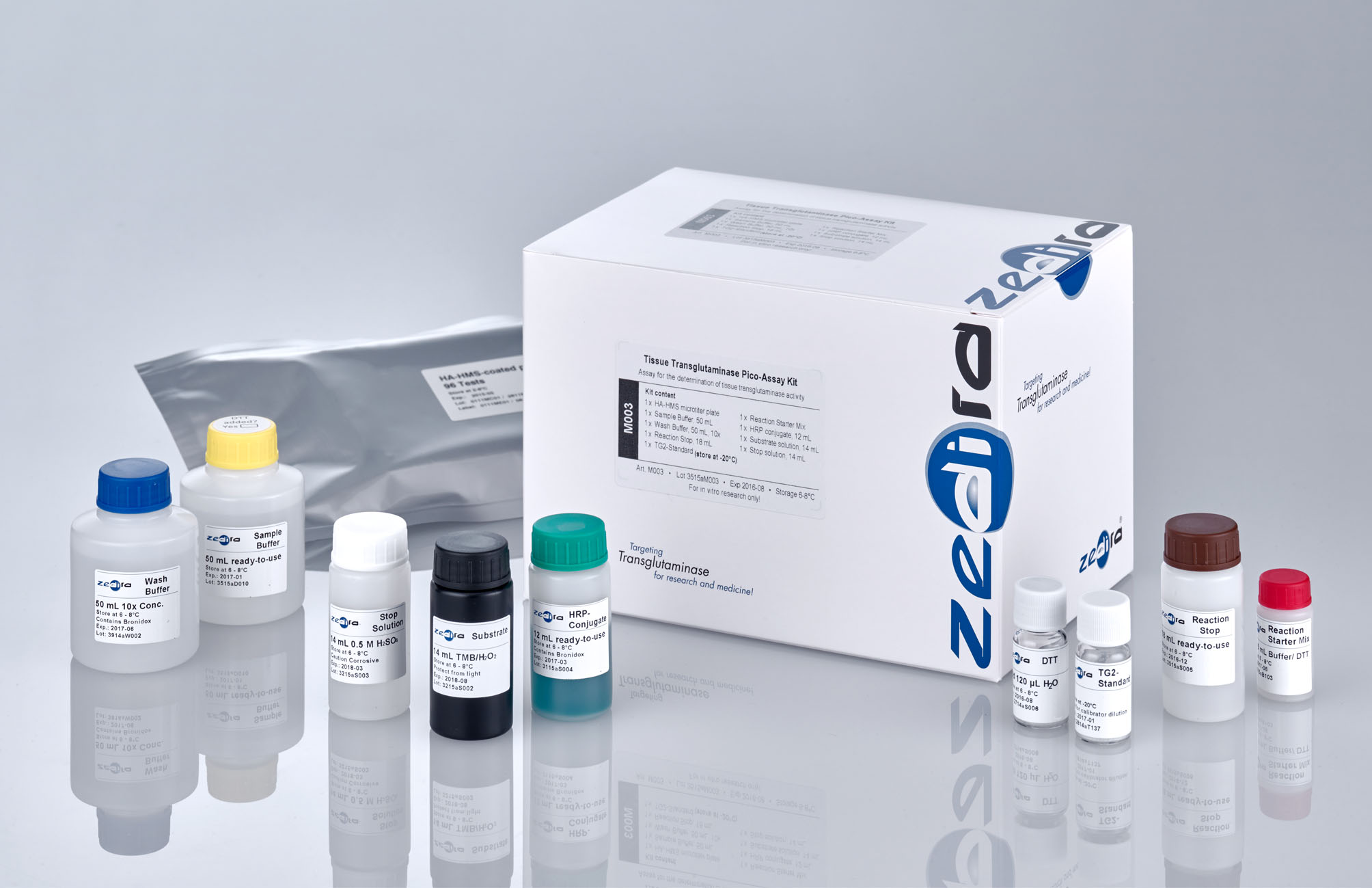
Tissue Transglutaminase Pico-Assay Kit
Assay also suitable for FXIII and TG3 Art. No.
M003
Background info
The Tissue Transglutaminase Pico-Assay Kit is based on a high activity high molecular weight substrate (HA-HMS) coated on a solid phase as glutamine-donor and a biotinylated substrate of transglutaminase (Biotinyl-cadaverine) as primary amine. Samples suspected of containing TG2 are incubated with calcium, dithiothreitol (DTT) and biotinyl-cadavarine in the wells of microtiter plates.

Specificity
The kit is selective, but not specific for tissue transglutaminase i.e. it also measures FXIII and TG3 activity in samples. Please contact us if calibration for TG3 or FXIII are required. TG1 cannot be measured with this kit.
Assay principle
The wells of the solid phase are coated with a specially developed high activity high molecular weight transglutaminase substrate (HA-HMS).
1st reaction: Tissue transglutaminase present in the sample covalently links biotinyl-cadaverine to the high molecular weight substrate (HA-HMS) coated on the microtiter plate surface.
2nd reaction: Streptavidin conjugated to horse-radish peroxidase (HRP) binds to the biotinyl-group incorporated into the HA-HMS solid phase.
3rd reaction: HRP converts a substrate (TMB) into a blue product which upon addition of the stop solution turns yellow. Samples containing transglutaminase activity develop the blue colour (which upon addition of the stop solution turns yellow), whereas samples without activity remain colourless.
1st reaction: Tissue transglutaminase present in the sample covalently links biotinyl-cadaverine to the high molecular weight substrate (HA-HMS) coated on the microtiter plate surface.
2nd reaction: Streptavidin conjugated to horse-radish peroxidase (HRP) binds to the biotinyl-group incorporated into the HA-HMS solid phase.
3rd reaction: HRP converts a substrate (TMB) into a blue product which upon addition of the stop solution turns yellow. Samples containing transglutaminase activity develop the blue colour (which upon addition of the stop solution turns yellow), whereas samples without activity remain colourless.
Reagents in the kit
Microtiter plate
Sample Buffer
Wash Buffer
TG2-Standard for preparation of calibrators
DTT
Reaction Starter Mix
Reaction Stop
Streptavidin-HRP conjugate
Substrate solution
Stop solution
Directions for use
Sample Buffer
Wash Buffer
TG2-Standard for preparation of calibrators
DTT
Reaction Starter Mix
Reaction Stop
Streptavidin-HRP conjugate
Substrate solution
Stop solution
Directions for use
Application
Assay for the determination of Tissue Transglutaminase (TG2) activity in the picogram scale
Intended use
The present kit is intended for the quantitative determination of tissue transglutaminase activity in a broad application diversity. The kit can also be used for screening of transglutaminase inhibitors.
Storage
Store the remaining kit at 4 - 8°C. It is stable up to the expiry date stated on the label of the box. Do not use kit beyond its expiry date. After opening the pouch keep unused microtiter wells resealed to minimize exposure to moisture.
Note
Intended for research use only, not for use in human, therapeutic or diagnostic applications.
 Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering  Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor  Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation