21. February 2020
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
by Philipp Spycher (February 2020)
Antibody-drug conjugates (ADCs) consist of three components: the antibody (mediates tumor-targeting) the payload (drug) and the linking-moiety (linker) that connects the drug to the antibody. ADCs are thought to selectively deliver drugs to tumors or other targets while the healthy tissue is spared, enabling thereby a so-called targeted chemotherapy.
Despite this simple concept, the field has faced major challenges with regard to efficacy and safety. In recent years, tremendous progress has been made leading to 7 approved ADCs and numerous ADCs to be approved in near future. Still, current ADCs and ADC technologies face three major challenges: 1) challenging and costly ADC assembly, 2) linker instability leading to unacceptable toxicities and reduced efficacy, 3) limited solubility of the linker resulting in non-optimal drug properties. In fact, many of the clinical ADC failures could be attributed to one or more of the above mentioned weaknesses.
The Araris difference
At Araris Biotech AG we are developing a novel linker technology to address these challenges. Our technology is based on novel lysine-peptide linkers and microbial transglutaminase (MTG) -mediated conjugation to the antibody. Using our lysine-peptide linkers we can conjugate the payload directly to the Q295 residue of fully glycosylated (native) antibodies without any prior antibody engineering step. The hydrophilic nature of our linkers furthermore helps to accommodate the (often) hydrophobic payloads and keep them well-solubilized resulting in ADCs with favorable physicochemical properties.
The conjugation can be done in 1-step whereby the linker-payload is directly conjugated to the antibody (Fig 1A, upper panel). Alternatively, the payload can be conjugated in two steps with the conjugation chemistry being flexible, like azide(N3)-click-chemistry or thiol-maleimide (Fig. 1A, lower panel). Due the peptidic nature of the linker, the drug-load can be easily increased from two (DAR2) to four attachment sites (DAR4) using a branched linker with a single-attachment site on the native antibody (Fig. 1B). Different conjugation chemistries can also be provided on the same linker, like azide and thiol, enabling thus the conjugation of two different payloads (DAR 2+2) (Fig. 1B).
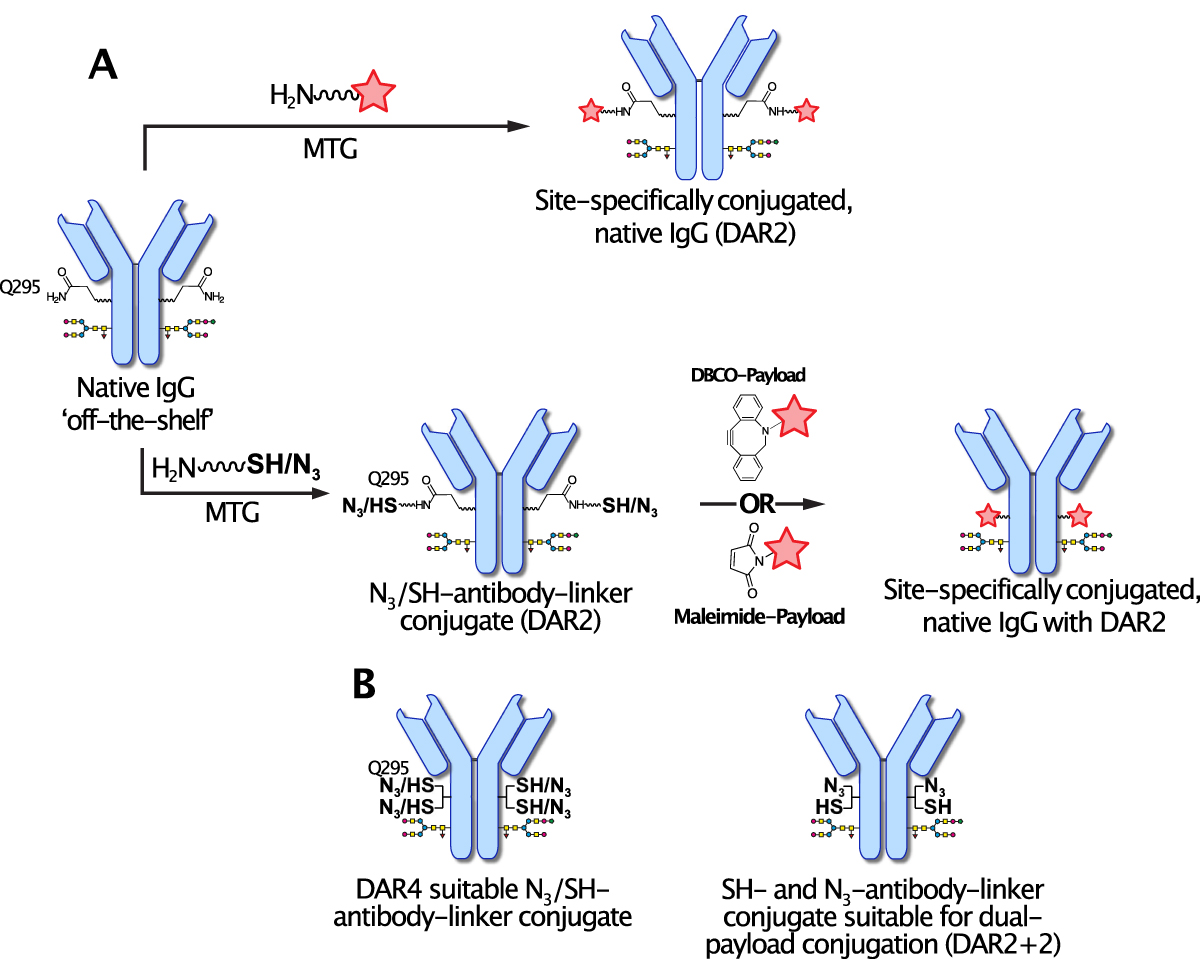
Fig. 1. Using a peptide-linker containing a lysine residue enables site-specific MTG-mediated conjugation to Q295 of native antibodies without prior antibody engineering. The payload can be conjugated in a 1- or 2-step process, by either conjugating the linker-payload to the antibody or first installing a reactive handle on the antibody followed by payload conjugation (A). The peptidic nature thereby allows to install various chemistries such as azide (N3) for click-chemistry or thiols for maleimide conjugation. By branching the linker two conjugation sites can be incorporated enabling thus a drug-load of 4 (DAR4) using e.g. N3 or Thiol (B). Both types of chemistries can also be installed on the same linker for dual-payload conjugation (DAR2+2).
In summary, our results indicate that the ADCs generated with our linker technology have favorable biophysical properties, are stable and result in very efficient anti-tumor responses in efficacy studies.
Thus, ADC generation with the Araris Linker Technology, offers the advantage that any native and unmodified antibody can be used ‘off-the-shelf’. ADCs can be generated in as little as 48 hours and are highly stable and efficacious. Due to the hydrophilic nature of the linker and its chemical versatility it is well suited to accommodate all payloads. At Araris we thus believe that we can offer ADCs to patients with an improved therapeutic window.
The author:

Antibody-drug conjugates (ADCs) consist of three components: the antibody (mediates tumor-targeting) the payload (drug) and the linking-moiety (linker) that connects the drug to the antibody. ADCs are thought to selectively deliver drugs to tumors or other targets while the healthy tissue is spared, enabling thereby a so-called targeted chemotherapy.
Despite this simple concept, the field has faced major challenges with regard to efficacy and safety. In recent years, tremendous progress has been made leading to 7 approved ADCs and numerous ADCs to be approved in near future. Still, current ADCs and ADC technologies face three major challenges: 1) challenging and costly ADC assembly, 2) linker instability leading to unacceptable toxicities and reduced efficacy, 3) limited solubility of the linker resulting in non-optimal drug properties. In fact, many of the clinical ADC failures could be attributed to one or more of the above mentioned weaknesses.
The Araris difference
At Araris Biotech AG we are developing a novel linker technology to address these challenges. Our technology is based on novel lysine-peptide linkers and microbial transglutaminase (MTG) -mediated conjugation to the antibody. Using our lysine-peptide linkers we can conjugate the payload directly to the Q295 residue of fully glycosylated (native) antibodies without any prior antibody engineering step. The hydrophilic nature of our linkers furthermore helps to accommodate the (often) hydrophobic payloads and keep them well-solubilized resulting in ADCs with favorable physicochemical properties.
The conjugation can be done in 1-step whereby the linker-payload is directly conjugated to the antibody (Fig 1A, upper panel). Alternatively, the payload can be conjugated in two steps with the conjugation chemistry being flexible, like azide(N3)-click-chemistry or thiol-maleimide (Fig. 1A, lower panel). Due the peptidic nature of the linker, the drug-load can be easily increased from two (DAR2) to four attachment sites (DAR4) using a branched linker with a single-attachment site on the native antibody (Fig. 1B). Different conjugation chemistries can also be provided on the same linker, like azide and thiol, enabling thus the conjugation of two different payloads (DAR 2+2) (Fig. 1B).

Fig. 1. Using a peptide-linker containing a lysine residue enables site-specific MTG-mediated conjugation to Q295 of native antibodies without prior antibody engineering. The payload can be conjugated in a 1- or 2-step process, by either conjugating the linker-payload to the antibody or first installing a reactive handle on the antibody followed by payload conjugation (A). The peptidic nature thereby allows to install various chemistries such as azide (N3) for click-chemistry or thiols for maleimide conjugation. By branching the linker two conjugation sites can be incorporated enabling thus a drug-load of 4 (DAR4) using e.g. N3 or Thiol (B). Both types of chemistries can also be installed on the same linker for dual-payload conjugation (DAR2+2).
In summary, our results indicate that the ADCs generated with our linker technology have favorable biophysical properties, are stable and result in very efficient anti-tumor responses in efficacy studies.
Thus, ADC generation with the Araris Linker Technology, offers the advantage that any native and unmodified antibody can be used ‘off-the-shelf’. ADCs can be generated in as little as 48 hours and are highly stable and efficacious. Due to the hydrophilic nature of the linker and its chemical versatility it is well suited to accommodate all payloads. At Araris we thus believe that we can offer ADCs to patients with an improved therapeutic window.
The author:

Philipp Spycher obtained his Master’s Degree and Ph.D. from ETH Zurich (Switzerland) at the interface of Material Science and Protein Engineering. During his post-doctoral work at the Paul Scherrer Institute (PSI, Switzerland), he introduced the novel approach using transglutaminases for antibody conjugation that led to the discovery of the Araris Linker Technology. Philipp won the PSI Founder Fellowship as well as several other prices and grants to commercialize the technology. He is now leading Araris as CEO and managed to assemble a world-class team of co-founders and co-workers.
 Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering  Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor  Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation