Drug candidates developed by Zedira:
ZED1227
Proof of concept was shown in a Phase 2a study on celiac disease
Proof of concept was shown in a Phase 2a study on celiac disease
- All dose groups met the primary endpoint
- Placebo-like safety profile
- First-in-class compound
- Validation of a transglutaminase as a druggable target for the first time
Partnering opportunity:
Licensed to Takeda Pharmaceuticals and Dr. Falk Pharma

ZED3269
- Novel 2nd generation tissue transglutaminase inhibitor
- Improved oral bioavailability
- Reversible-acting warhead
- Rodent pharmacokinetic and efficacy data available
Seeking licensee(s)/development partner(s)
Preferred indications include
ZED3197
- First-in-class “safe” anticoagulant
- Direct-acting irreversible Factor XIIIa (F13a) inhibitor
- Proof-of-principle in rabbit model shown
Seeking licensee(s)/development partner(s)
Preferred indications include
Zedira is open to considering alliances and agreements with the pharmaceutical industry. Please do not hesitate to contact us. Zedira’s management team, Dr. Martin Hils and Dr. Ralf Pasternack, will be pleased to be at your disposal:
| Phone: | +49 6151 66628-0 or | |
| E-mail: | contact@zedira.com |
 Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering 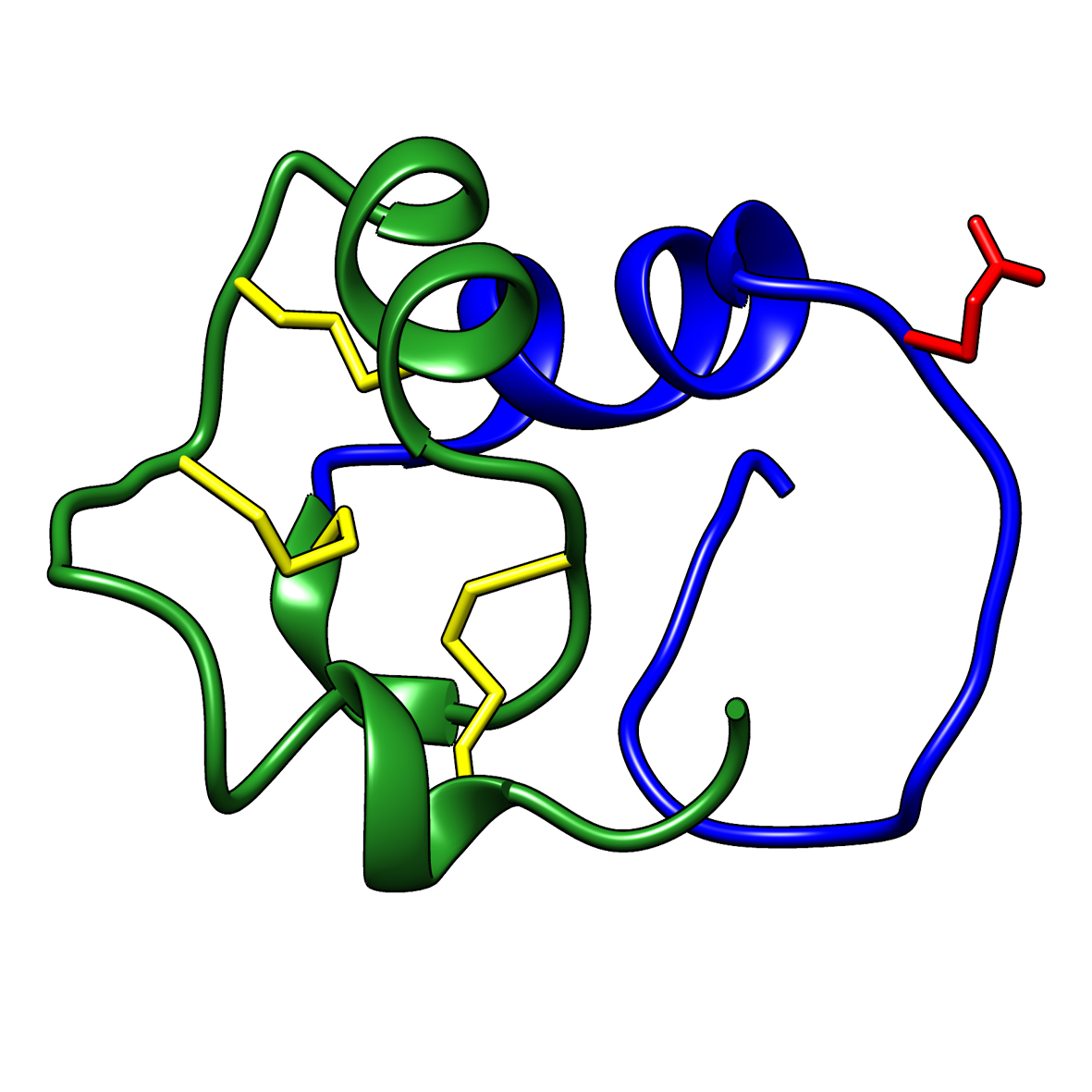 Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor 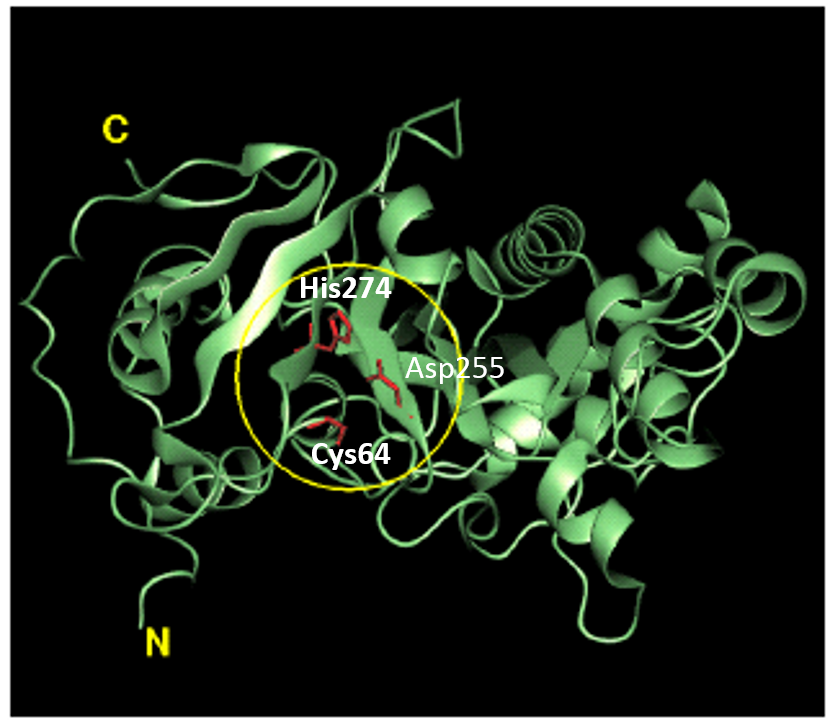 Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation