Back to all products
Product criteria
Product criteria
| Substance |
|---|
| Michael acceptor 3 |
Michael acceptor
peptidomimetics
Zedira designed peptidomimetic blockers bearing a Michael acceptor warhead.
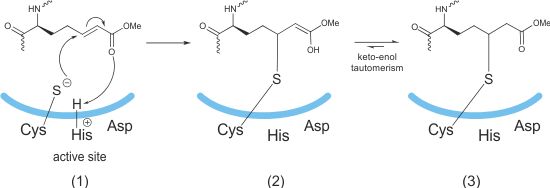
The electrophilic α,β-unsaturated carbonyl compounds are attacked by the active site cysteine residue (1) to form an irreversible complex (2,3).
The peptidic lead compound Z013 (ZED754) was co-crystallized with tissue transglutaminase. The picture shows the binding mode to the active site. The scaffold was subsequently optimized using medicinal chemistry.
Our clinical candidate ZED1227 is the first TG inhibitor in the clinics.
Compound ZED1301 (A108) is a potent factor XIII inhibitor.
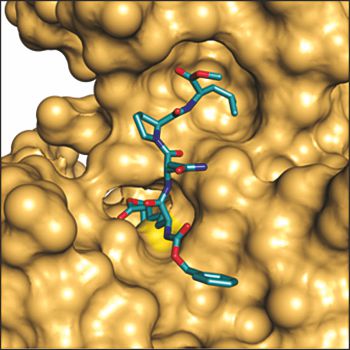
Structure of inhibited tissue transglutaminase.
The inhibitor (shown in cyan) is covalently bound by a Michael addition reaction to the active site Cys 277 (yellow surface), which is located in the catalytic tunnel. It mimics the peptide substrate. The main part of the inhibitor fits onto the protein surface, forming several hydrogen bonds and stabilizing the open conformation
(Lindemann et al., poster presentation at XX Int. Med. Chem. Symp. 2008).
The electrophilic α,β-unsaturated carbonyl compounds are attacked by the active site cysteine residue (1) to form an irreversible complex (2,3).

The peptidic lead compound Z013 (ZED754) was co-crystallized with tissue transglutaminase. The picture shows the binding mode to the active site. The scaffold was subsequently optimized using medicinal chemistry.
Our clinical candidate ZED1227 is the first TG inhibitor in the clinics.
Compound ZED1301 (A108) is a potent factor XIII inhibitor.

Structure of inhibited tissue transglutaminase.
The inhibitor (shown in cyan) is covalently bound by a Michael addition reaction to the active site Cys 277 (yellow surface), which is located in the catalytic tunnel. It mimics the peptide substrate. The main part of the inhibitor fits onto the protein surface, forming several hydrogen bonds and stabilizing the open conformation
(Lindemann et al., poster presentation at XX Int. Med. Chem. Symp. 2008).
| Art. No. | Name | Unit | Price |
|---|---|---|---|
| A108 | Ac-(D)-Asp-MA-Nle-Nle-Leu-Pro-Trp-Pro-OH, "ZED1301" | 5 mg | 550 € |
| B015 | Biotin-Ahx-MA-QPL-OMe | 10 mg | 495 € |
| Z013 | Z-MA-QPL-OMe | 10 mg | 495 € |
 Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering  Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor  Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation