Zedira’s lead indication is celiac disease. Celiac disease is the most common chronic inflammation of the small intestine. The autoimmune disease affects up to 2% of most populations and is caused by nutritional gluten in genetically predisposed individuals. A key step in celiac disease pathogenesis is gluten deamidation and immunogenic potentiation catalyzed by the patient’s own tissue transglutaminase in the gut.
The small molecule ZED12271 targets the dysregulated transglutaminase within the small intestine to prevent the immune response to transglutaminase-modified gluten, which drives the disease process. Blocking tissue transglutaminase has the potential to offer patients additional safety when used in conjunction with a ‘largely’ gluten-free diet, thereby improving the quality of life of millions of people.
The drug candidate ZED1227 is a “first-in-class” compound, meaning it is the first transglutaminase blocker explored in humans. In 2011, Dr. Falk Pharma licensed the rights for ZED1227 in Europe and took charge of the preclinical and clinical development of the new chemical entity towards a pharmacological agent. In July 2021, Dr. Falk Pharma and Zedira announced the “successful completion of the phase 2a proof of concept study of ZED1227 for the treatment of Celiac Disease". The detailed results of the study are published in the New England Journal of Medicine (https://www.nejm.org/doi/full/10.1056/NEJMoa2032441). The press release for an overview can be found here. In 2022 Takeda Pharmaceuticals entered collaboration and licensing agreement with Zedira and Dr. Falk Pharma (press release).
The proof-of-concept study is of vital importance beyond celiac disease:
The first-in-class new chemical entity validated TG2 as a druggable target. Now, the spotlight focuses on fibrotic disorders and anticoagulation.
1 Büchold C, Hils M, Gerlach U, Weber J, Pelzer C, Heil A, Aeschlimann D, Pasternack R. Features of ZED1227: The First-In-Class Tissue Transglutaminase Inhibitor Undergoing Clinical Evaluation for the Treatment of Celiac Disease. Cells. 2022; 11(10):1667. https://doi.org/10.3390/cells11101667
 Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible? 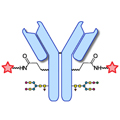 Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering 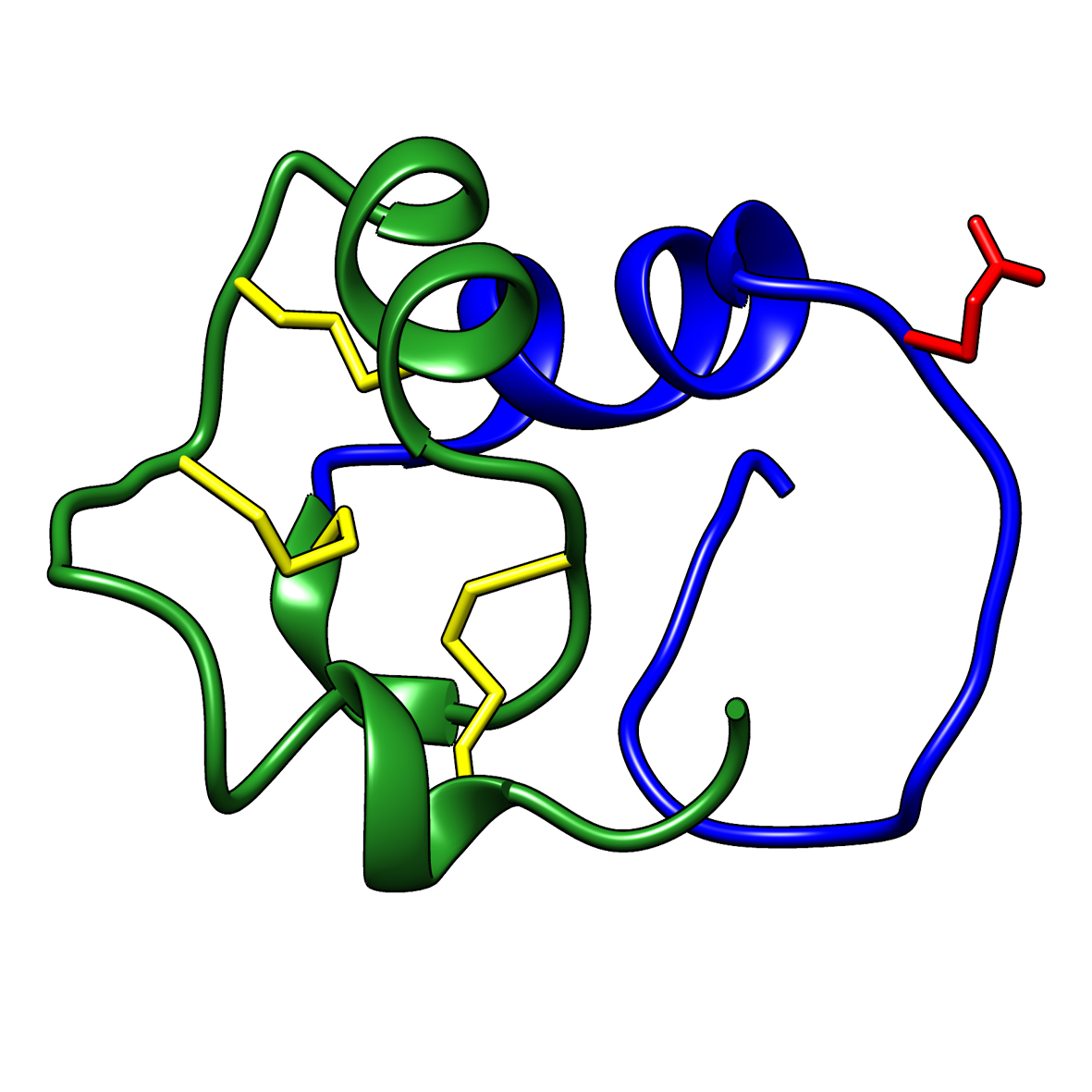 Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor 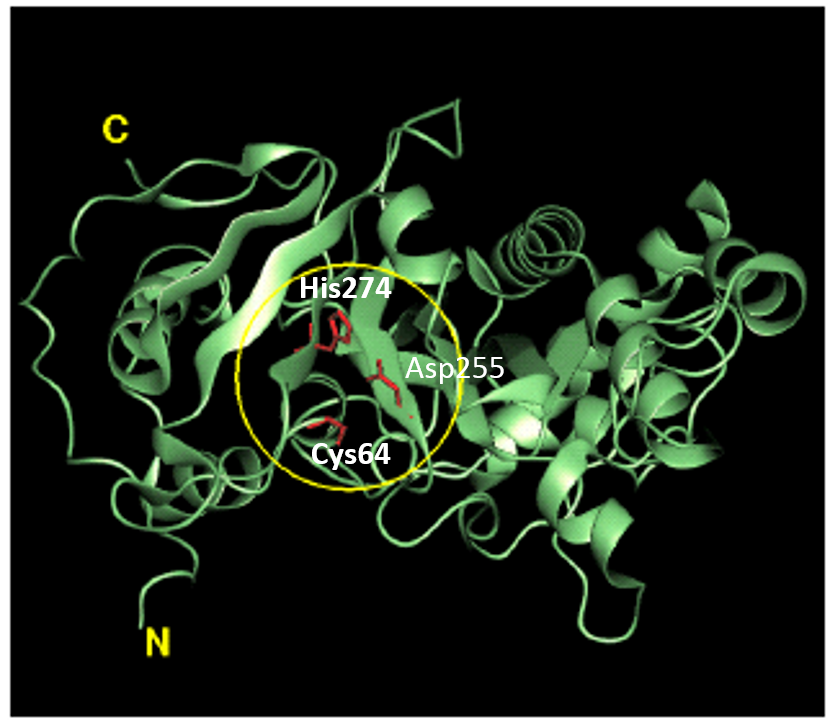 Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation