Back to all products
| TGase Protein Labeling Kits |
| Transglutaminase |
| Reaction stopper |
| Amine-donors |
| Glutamine-donor peptides |
| TGase Labeling Service |
Protein Labeling
Protein labeling using transglutaminase (TGase) is a smart enzymatic labeling alternative to chemical protein labeling procedures.
Protein biotinylation, PEGylation or site-directed protein labeling with fluorescent dyes are common examples of TGase labeling.
Zedira’s protein labeling tools are available as

Advantages of transglutaminase (TGase) mediated site-specific protein labeling:
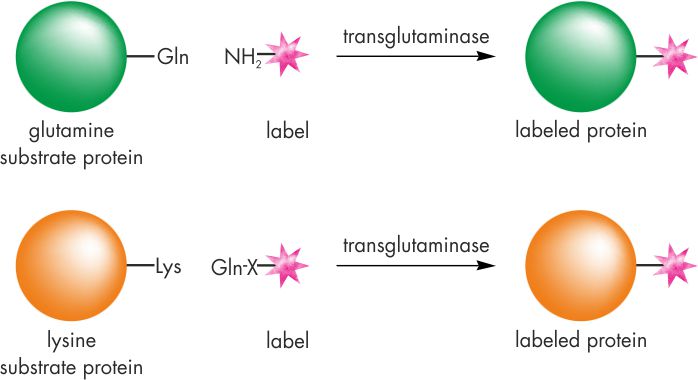
Principle of TGase-catalyzed protein labeling, protein modification, and protein conjugation
TGases catalyze the acyl transfer reaction between the γ-carboxamide group of protein-bound glutamine residues and the ε-amino group of protein-bound lysine or primary amines. Thus, primary amines as well as glutamine-containing peptides may carry a broad variety of labels like biotin, PEG or fluorescent dyes.
Requirements for transglutaminase labeling
Transglutaminase protein labeling requires substrate sequences on the target protein surface, which are generally not abundant on proteins:
Examples for utilization of modified proteins
Please find information about TGase Protein Labeling Kits, the individual components, and Zedira’s TGase Labeling Service in the respective categories.
In case of any questions just contact us!
![]()
Protein biotinylation, PEGylation or site-directed protein labeling with fluorescent dyes are common examples of TGase labeling.
Zedira’s protein labeling tools are available as
- TGase Protein Labeling Kits
- Individual components
- TGase Labeling Service

Advantages of transglutaminase (TGase) mediated site-specific protein labeling:
- Defined degree of labeling
- Defined label position(s)
- Homogenously labeled protein
- Higher solubility in water
- Minimized amounts of unlabeled protein
- No or reduced impact on the bioactivities of labeled proteins
Principle of TGase-catalyzed protein labeling, protein modification, and protein conjugation
TGases catalyze the acyl transfer reaction between the γ-carboxamide group of protein-bound glutamine residues and the ε-amino group of protein-bound lysine or primary amines. Thus, primary amines as well as glutamine-containing peptides may carry a broad variety of labels like biotin, PEG or fluorescent dyes.

Requirements for transglutaminase labeling
Transglutaminase protein labeling requires substrate sequences on the target protein surface, which are generally not abundant on proteins:
- Accessible lysine residues
- or accessible glutamine residues
- if no lysine or glutamin residues are accessible, transglutaminase substrate sequence tags can be introduced recombinantly
Examples for utilization of modified proteins
- Fluorescence-based HTS-assay
- Diagnostics (antibodies, antibody-binding proteins, lectins etc.)
- Therapeutic proteins (PEGylation)
- Protein immobilization (after biotinylation)
Please find information about TGase Protein Labeling Kits, the individual components, and Zedira’s TGase Labeling Service in the respective categories.
In case of any questions just contact us!
| Phone: | +49 6151 66628-0 | |
| E-mail: | contact@zedira.com |
 Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering  Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor 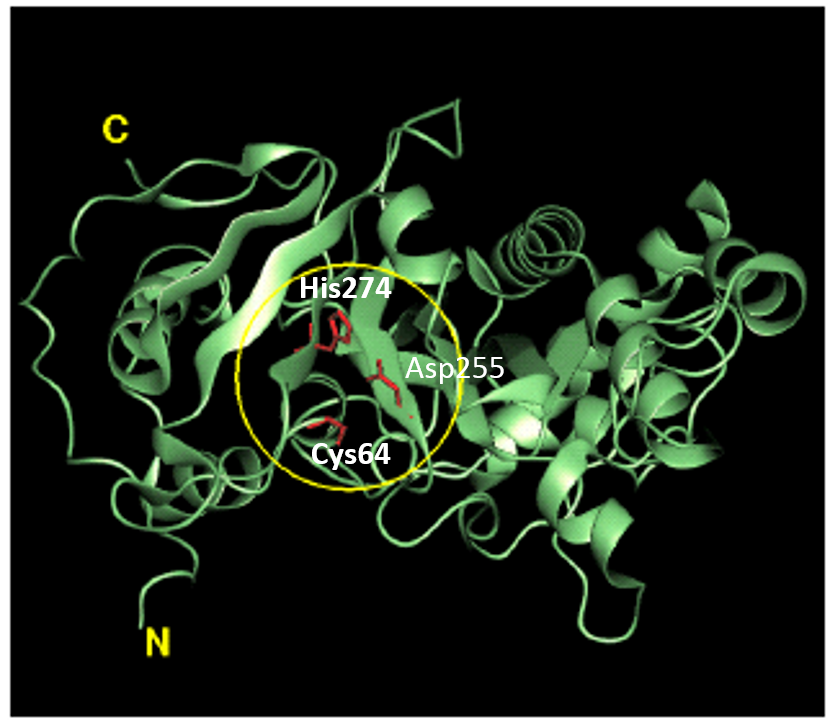 Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation? 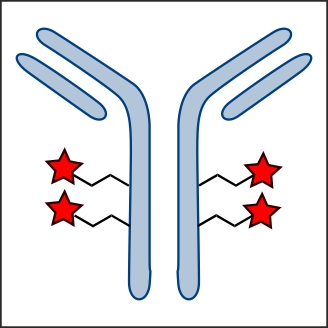 Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation