Drug Discovery targeting coagulation Factor XIIIa (F13a)
Blocking Coagulation Factor XIIIa (FXIIIa) in at-risk patients
The physiological functions of coagulation factor XIIIa - also called plasma transglutaminase - are well known. Factor XIII is activated by thrombin to factor XIIIa and subsequently cross-links fibrin fibers. This covalent cross-linking reaction augments blood clot strength while also improving the visco-elastic properties. Further, the clot is covalently decorated with α2-antiplasmin, increasing clot stability against premature fibrinolysis by plasmin. In summary, factor XIII is the major factor influencing clot stability, clot maturation, and clot lysis.
Using a rabbit animal model, Zedira showed the proof-of-principle of “safe” anticoagulation therapy by the inhibition of factor XIIIa published in the Journal of Thrombosis and Haemostasis: “Novel inhibitor ZED3197 as potential drug candidate in anticoagulation targeting coagulation FXIIIa (F13a)”.
https://onlinelibrary.wiley.com/doi/full/10.1111/jth.14646.
The crucial difference compared with the existing anticoagulants lies in the fact that a soft and easily degradable blood clot can still be formed while thrombin generation – and therefore platelet activation – is not affected.
A significant reduction in the life-threatening tendency to bleed as provoked by current drugs is thus likely to be achieved. Today about 50% of patients are excluded from anticoagulation therapy due to the risk of bleeding events.
Zedira’s factor XIIIa blockers have the highest efficacy demonstrated to date. Our compounds have the potential to provide a novel therapeutic option with a superior benefit/risk ratio in hypercoagulable disease states.
 Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible? 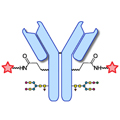 Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering 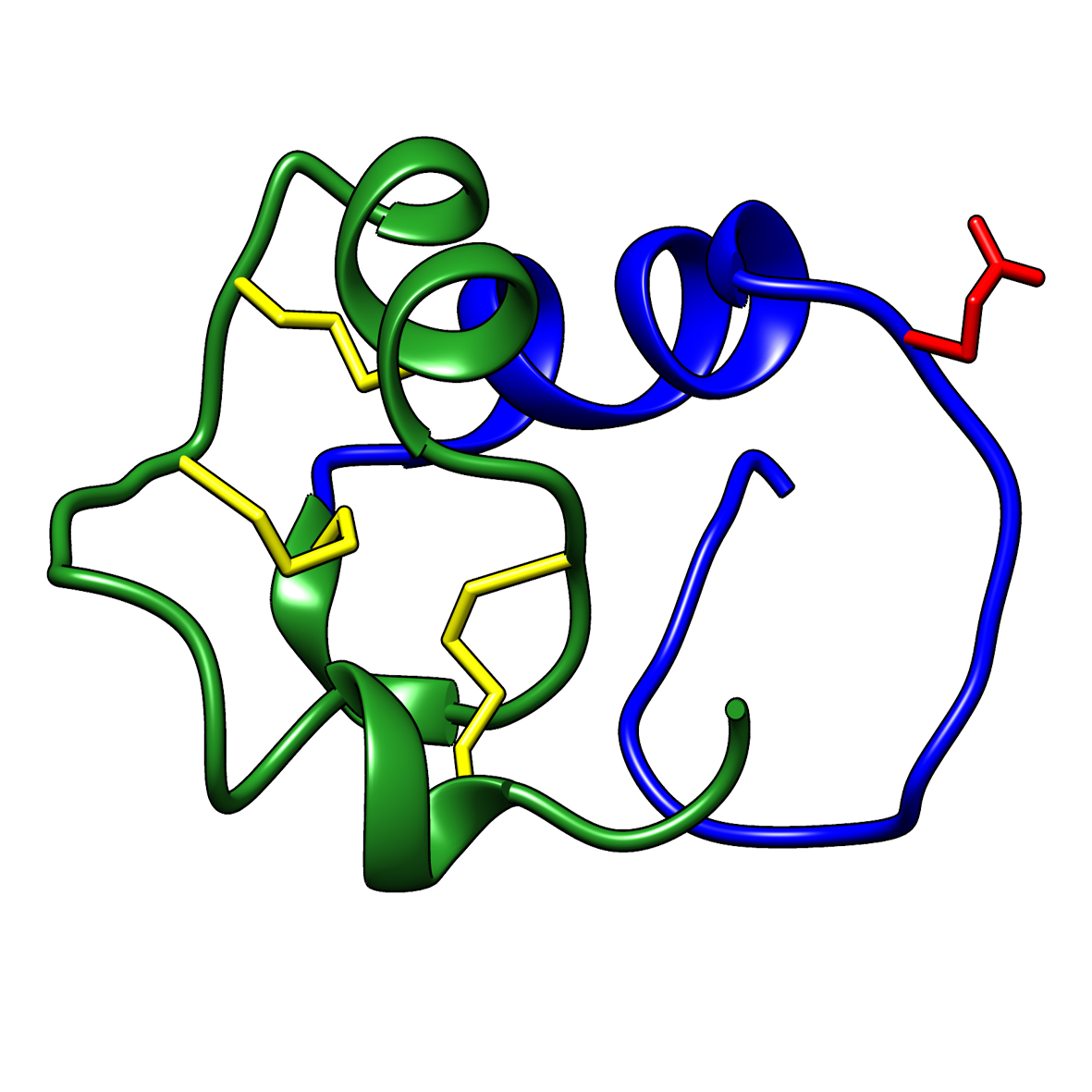 Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor 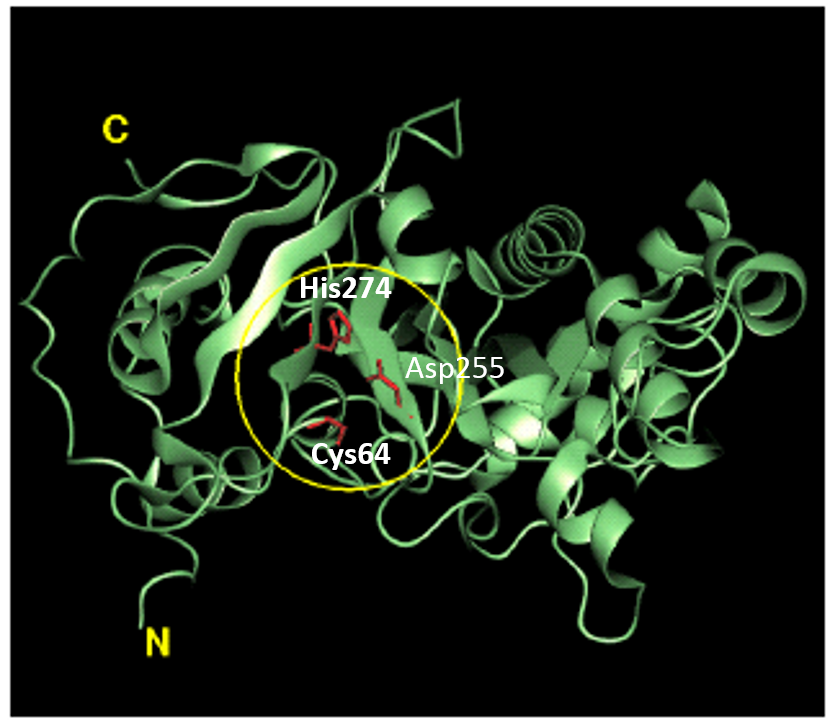 Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation? 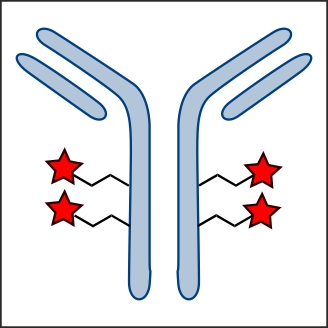 Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation