Back to all products
| Isopeptidase-Fluorogenic Assays |
| Transglutaminase fluorogenic Activity Assay |
| Photometric Activity Assay |
| Enzyme Immuno Assay |
| Glutamine-donor peptides |
| Amine-donors |
| Protein Substrates |
| „Hitomi“-peptides |
Photometric Activity Assay
ZediExclusive Tissue Transglutaminase Assay Kit
(Chromogenic activity assay optimized for tissue transglutaminase, also suitable for epidermal transglutaminase) Art. No.
Z010
Background info
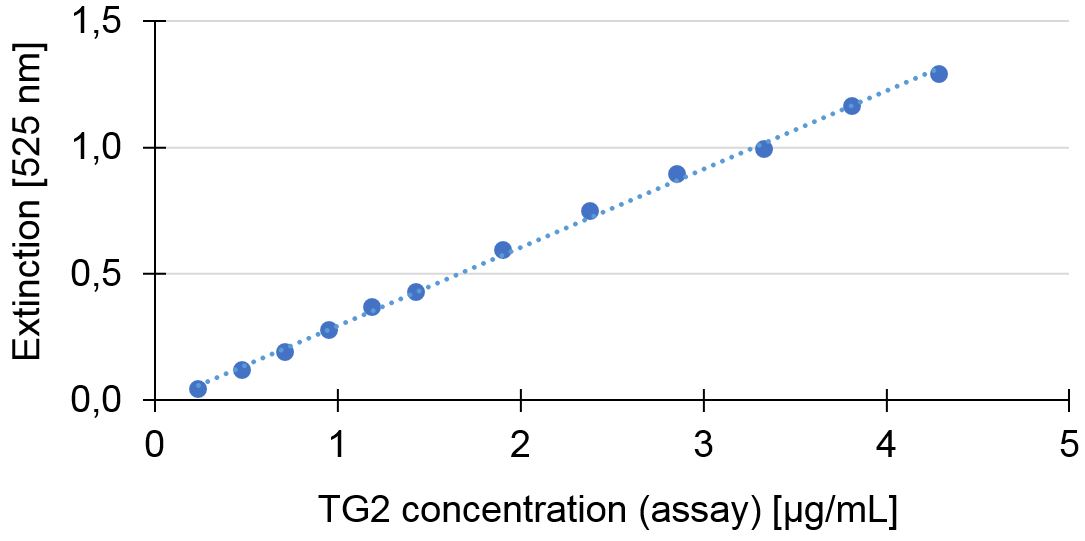
TG2 is present in various tissues and involved in a plentitude of physiological as well as pathological processes. The enzyme catalyses the acyl transfer reaction between the γ-carboxyamide group of peptidebound glutamine residues and a variety of primary amines, particularly the ε-amino group of lysine (Lorand L. et al., 1962). This assay enables the measurement of TG2 activity according to the chromogenic hydroxamate detection principle (Grossowicz, N. et al., 1950).
Assay principle
The TISSUE TRANSGLUTAMINASE ASSAY KIT uses Z-QQPF as the amine acceptor substrate and hydroxylamine as amine donor. In the presence of tTG hydoxylamine is incorporated into Z-QQPF to form Z-glutamylhydroxamate-QPF which develops a colored complex with iron (III) detectable at 525 nm.
Reagents in the kit
(1A) ACTIVITY REAGENT 1A: lyophilized MOPS buffer pH 7.6 containing Z-QQPF, DTT and calcium, 3 vials
(2A) ACTIVITY REAGENT 2A: Hydroxylamine, 3 vials
(3S) STOP REAGENT 3S: Hydrochloric acid [4% v/v], Iron (III) chloride, 3 vials
(4P) TG2 POSITIVE CONTROL 4P: lyophilized TG2, 3 vials
(2A) ACTIVITY REAGENT 2A: Hydroxylamine, 3 vials
(3S) STOP REAGENT 3S: Hydrochloric acid [4% v/v], Iron (III) chloride, 3 vials
(4P) TG2 POSITIVE CONTROL 4P: lyophilized TG2, 3 vials
Equipment
The TISSUE TRANSGLUTAMINASE ASSAY KIT can be used in standard spectrophotometers with polystyrene 1 mL cuvettes or MTP. Refer to the instructions of the manufacturer.
Intended use
Determination of tissue transglutaminase activity (tTG, TG2). Kit is sufficient for 3 x 11 measurements in cuvettes or 3 x 36 measurements in 96 well microtiter plates (MTP).
Storage
4P has to be stored at -20°C (shipment is possible at 4 – 8°C).
1A, 2A, 3S has to be stored at 4 – 8°C. The unopened reagents are at least stable until the expiration date printed on the box.
1A, 2A, 3S has to be stored at 4 – 8°C. The unopened reagents are at least stable until the expiration date printed on the box.
Note
INTENDED FOR RESEARCH USE ONLY, NOT FOR USE IN HUMAN, THERAPEUTIC OR DIAGNOSTIC APPLICATIONS.
Related Products
 Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering  Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor  Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation