Back to all products
| Microbial (Pro)TGase |
| Inhibitor |
| Activity Assay Kits |
| ELISA |
| Antibodies |
| TGase Protein Labeling Kits |
Activity Assay Kits
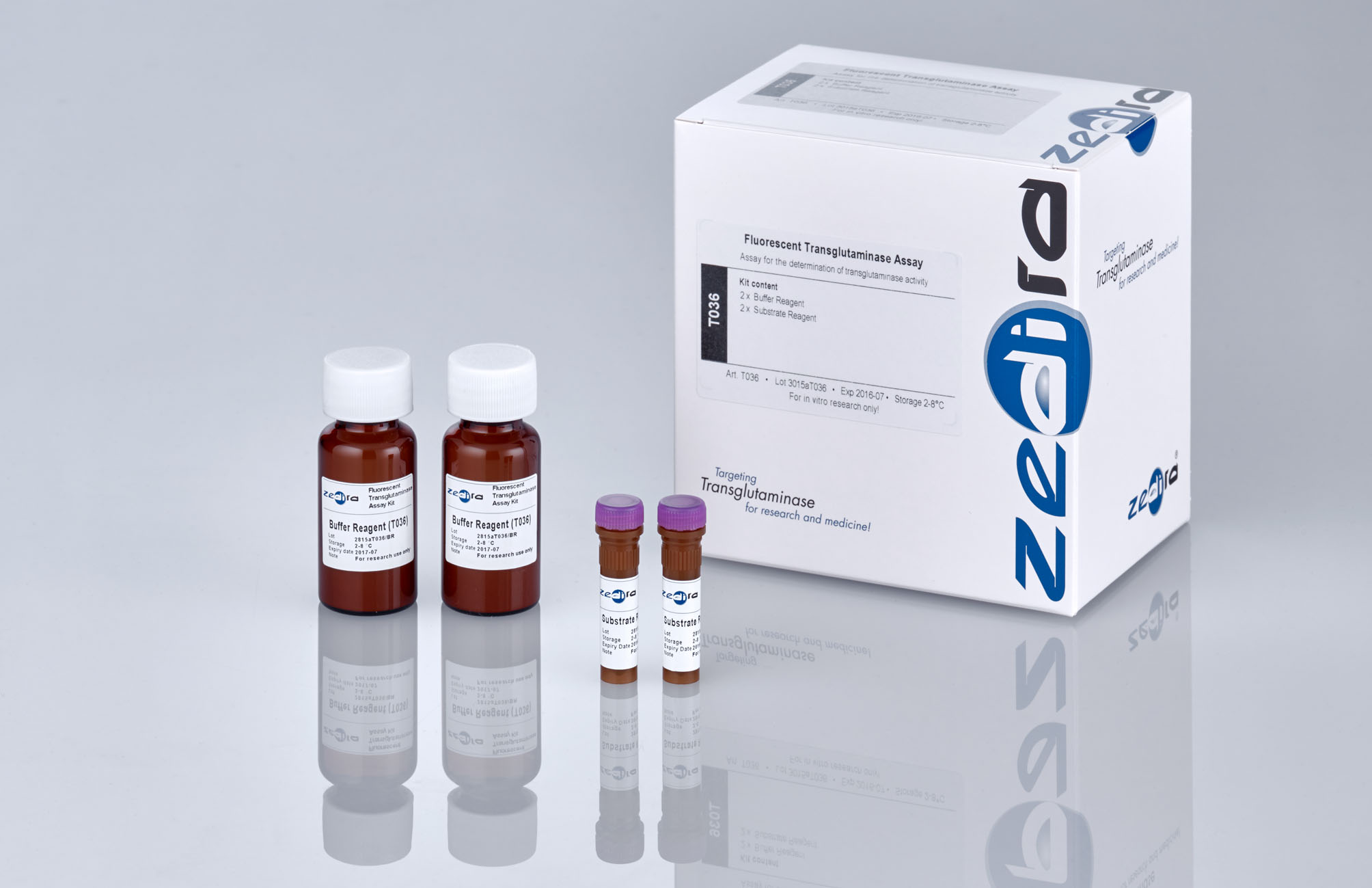
Transglutaminase Assay Kit ("DCC"), fluorescent
Casein, Dansylcadaverine Art. No.
T036
Description
Assay principle
Transglutaminases catalyze acyl transfer reactions from glutamine residues in proteins or peptides to primary amines. The transglutaminase-catayzed covalent coupling of monodansylcadaverine into N,N-dimethylcasein produces a shift in intensity and wavelength of fluorescence of the dansyl group. The transglutaminase activity can be monitored online by measurement of the fluorescence (excitation wavelength 332 nm; emission wavelength 500 nm). The relative transglutaminase activity is shown by increase of fluorescence intensity over time.
Reagents in the kit
(1) SUBSTRATE REAGENT: 2 x 0,185 mg monodansylcadaverine
(2) BUFFER REAGENT: 2 x 20 ml lyophilized TRIS buffer pH 8,0 containing calcium chloride, sodium chloride, N,N-dimethylcasein, glutathione.
(2) BUFFER REAGENT: 2 x 20 ml lyophilized TRIS buffer pH 8,0 containing calcium chloride, sodium chloride, N,N-dimethylcasein, glutathione.
Equipment
The Fluorescent Transglutaminase Assay can be used in standard fluorescence spectrophotometers. Refer to the instructions of the manufacturer.
Intended use
Determination of transglutaminase activity (hTG1, hTG2, hTG6).
Reference(s)
Lorand et al., Anal. Biochem. 1971, 44:221-31
Note
Intended for research use only, not for use in human, therapeutic or diagnostic applications.
 Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering  Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor  Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation