Link 
Successful ISO9001:2015 recertification
Link Zedira supports the Tampere Celiac Disease Symposium 2025
Link Co-authored article published in Nature Immunology: Transcriptomic analysis of intestine following administration of a transglutaminase 2 inhibitor to prevent gluten-induced intestinal damage in celiac disease
Link 
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Link 
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Link Co-authored article: The Oral Transglutaminase 2 Inhibitor ZED1227 Accumulates in the Villous Enterocytes in Celiac Disease Patients during Gluten Challenge and Drug Treatment
Link Takeda Enters Collaboration and Licensing Agreement with Zedira and Dr. Falk Pharma to Develop First-in-Class Celiac Disease Therapy
Link Kooperations- und Lizenzvereinbarung zwischen Takeda, Zedira und Dr. Falk Pharma zur Entwicklung einer First-in-Class-Therapie für die Zöliakie
Link Co-authored posters at ICDS 2022
Link 
Successful ISO9001:2015 recertification
Link Dr. Falk Pharma and Zedira announce start of the phase 2a proof of concept study of ZED1227 for the treatment of non-alcoholic fatty liver disease (NAFLD)
Link Zedira publication - Features of ZED1227: The First-In-Class Tissue Transglutaminase Inhibitor Undergoing Clinical Evaluation for the Treatment of Celiac Disease
Link Dr. Falk Pharma and Zedira announce start of the phase 2b real-life study of ZED1227 for the treatment of Celiac Disease
Link 
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Link 
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Link 
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis

 Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible? 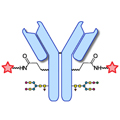 Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering 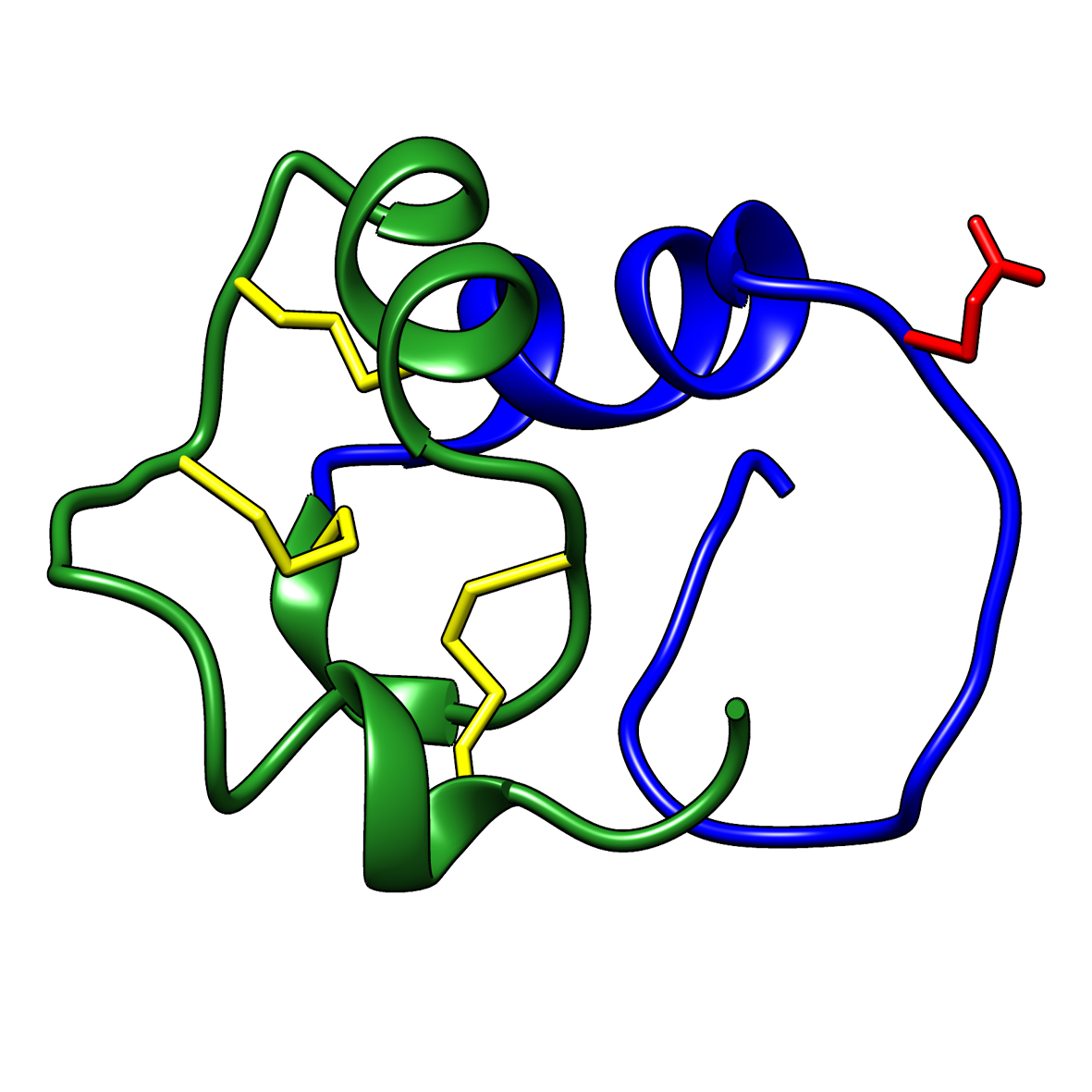 Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor 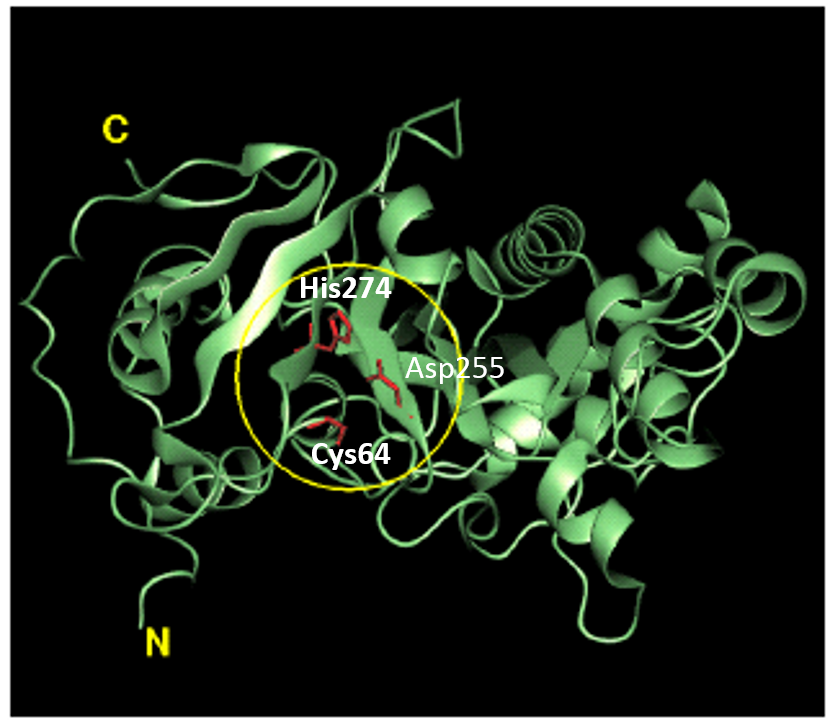 Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation? 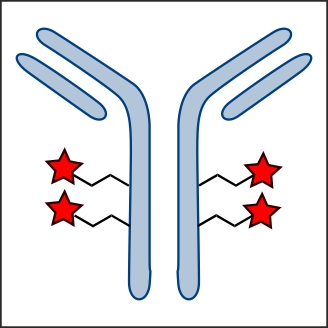 Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation