26. August 2019

 Transglutaminase-Newsletter | August 2019
Transglutaminase-Newsletter | August 2019

Paving the way to a new automated FXIII activity assay!
Just recently, Technoclone (Vienna, Austria) and Zedira presented a joint poster at the 27th ISTH Congress in Melbourne, Australia. The scientists involved, Martina Leiter, Ralf Pasternack, Christian Büchold, and Nikolaus Binder concluded:
“The fully automated TECHNOFLUOR FXIII Activity assay run on the new Ceveron s100 haemostasis analyzer is an appropriate method for fast and accurate determination of this critical factor in the stabilization of blood clots. With a high degree of linearity over a wide assay range, this new assay shows excellent recovery of EQA samples, demonstrating high agreement with other FXIII activity assays on the market.”
In this context, please find below our reagents targeting FXIII (F13, plasma transglutaminase) flanked by selected coagulation products:
Selection of Factor XIII products

Drug Discovery at Zedira targeting FXIIIa
Zedira developed direct-acting FXIIIa blockers for safe anticoagulation. This unique and promising approach has the potential of a significant reduction in the life-threatening tendency to bleeding as provoked by current drugs. Zedira scientists used structure-assisted drug design to develop potent and selective compounds while deciphering the structure of active coagulation factor XIII.
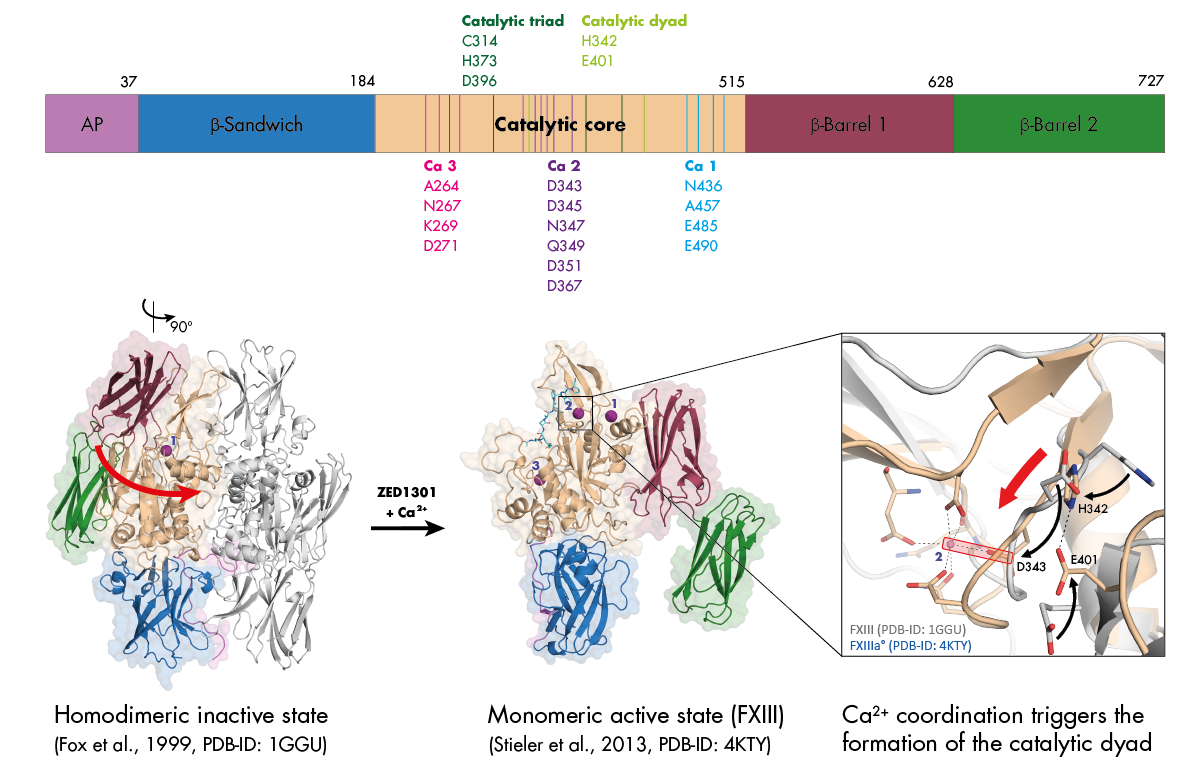
Structure of Active Coagulation Factor XIII
In the inactive state, recombinant FXIII exists as a dimer. Upon binding of the irreversible acting blocker ZED1301 (A108) and three calcium ions per subunit, FXIII dissociates and the ß-barrel 1 and ß-barrel 2 domains undergo a remarkable shift exposing the active site (upper left). The crystal structure of FXIII in its active conformation provides detailed information on an atomic level regarding the role of calcium in the activation process. Calcium coordination affects the shape of the active site of FXIII and triggers the formation of the catalytic dyad (H342, E401).
New automated FXIII activity assay

 Transglutaminase-Newsletter | August 2019
Transglutaminase-Newsletter | August 2019Paving the way to a new automated FXIII activity assay!
Just recently, Technoclone (Vienna, Austria) and Zedira presented a joint poster at the 27th ISTH Congress in Melbourne, Australia. The scientists involved, Martina Leiter, Ralf Pasternack, Christian Büchold, and Nikolaus Binder concluded:
“The fully automated TECHNOFLUOR FXIII Activity assay run on the new Ceveron s100 haemostasis analyzer is an appropriate method for fast and accurate determination of this critical factor in the stabilization of blood clots. With a high degree of linearity over a wide assay range, this new assay shows excellent recovery of EQA samples, demonstrating high agreement with other FXIII activity assays on the market.”
In this context, please find below our reagents targeting FXIII (F13, plasma transglutaminase) flanked by selected coagulation products:
Selection of Factor XIII products
Assays
| Art. No. | Name | Unit | Price |
| A101 | FXIII-Assay Substance, Abz-NE(CAD-DNP)EQVSPLTLLK-OH | 10 mg | 520 € |
| F001 | FXIII-Assay Kit | 1 Kit | 575 € |
Inhibitors
| Art. No. | Name | Unit | Price |
| T101 | 1,3,4,5-Tetramethyl-2[(2-oxo-propyl)thio] imidazolium chloride | 10 mg | 480 € |
| A108 | Ac-(D)-Asp-MA-Nle-Nle-Leu-Pro-Trp-Pro-OH | 5 mg | 480 € |
Special product
| Art. No. | Name | Unit | Price |
| G101 | Gly-Pro-Arg-Pro-amide | 25 mg | 370 € |
FXIII
| Art. No. | Name | Unit | Price |
| T027 | Human blood coagulation Factor XIII-A, recombinant | 200 µg | 370 € |
| T070 | Human Factor XIIIa, activated | 200 µg | 520 € |
| T050 | Human blood coagulation Factor XIII B subunit | 200 µg | 370 € |
New!
| Art. No. | Name | Unit | Price |
| T222 | Human alpha Thrombin, recombinant | 100 NIH Units | 185 € |
Antibodies
| Art. No. | Name | Unit | Price |
| A016 | Polyclonal antibody to human blood coagulation factor XIII (A-subunit) | 500 µg | 365 € |
| A032 | FITC-labeled polyclonal antibody to human factor XIII (A-subunit) | 200 µg | 395 € |
| A074 | Polyclonal antibody to human blood coagulation factor XIII (B-subunit) | 200 µg | 365 € |
| A077 | FITC-labeled polyclonal antibody to human factor XIII (B-subunit) | 200 µg | 395 € |
| A076 | DD-XLink-mab | 100 µg | 365 € |
Drug Discovery at Zedira targeting FXIIIa
Zedira developed direct-acting FXIIIa blockers for safe anticoagulation. This unique and promising approach has the potential of a significant reduction in the life-threatening tendency to bleeding as provoked by current drugs. Zedira scientists used structure-assisted drug design to develop potent and selective compounds while deciphering the structure of active coagulation factor XIII.

Structure of Active Coagulation Factor XIII
In the inactive state, recombinant FXIII exists as a dimer. Upon binding of the irreversible acting blocker ZED1301 (A108) and three calcium ions per subunit, FXIII dissociates and the ß-barrel 1 and ß-barrel 2 domains undergo a remarkable shift exposing the active site (upper left). The crystal structure of FXIII in its active conformation provides detailed information on an atomic level regarding the role of calcium in the activation process. Calcium coordination affects the shape of the active site of FXIII and triggers the formation of the catalytic dyad (H342, E401).
 Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira
Besuch des Bundesministers für Wirtschaft und Klimaschutz Dr. Robert Habeck bei der Zedira  Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!
Discover Our New Catalogue Edition and Dive into the World of Transglutaminases!  Successful ISO9001:2015 recertification
Successful ISO9001:2015 recertification  Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease
Dr. Falk Pharma and Zedira announce successful completion of the phase 2a proof-of-concept study of ZED1227 for the treatment of Celiac Disease  Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie
Dr. Falk Pharma und Zedira verkünden den erfolgreichen Abschluss der Phase 2a-Studie mit ZED1227 zur Behandlung von Zöliakie  Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis
Reversibly acting transglutaminase 2 inhibitors: drug candidates for the treatment of fibrosis  Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis
Transcriptomic analysis of the efficacy of TG2-inhibitor trials and human intestinal organoids modelling Celiac disease pathogenesis  Transglutaminase antibodies and neurological manifestations of gluten sensitivity
Transglutaminase antibodies and neurological manifestations of gluten sensitivity  Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?
Design of Oral FXIIIa Blockers as Safer Anticoagulants Mission Impossible?  Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering
Microbial transglutaminase (MTG) enables efficient and site-specific conjugation to native antibodies without the need of antibody engineering  Tridegin as FXIIIa inhibitor
Tridegin as FXIIIa inhibitor  Microbial transglutaminase: from discovery to market
Microbial transglutaminase: from discovery to market  Tissue transglutaminase inhibitors
Tissue transglutaminase inhibitors  Tissue transglutaminase in Alzheimers Disease
Tissue transglutaminase in Alzheimers Disease  Factor XIIIa: novel target for anticoagulation?
Factor XIIIa: novel target for anticoagulation?  Microbial transglutaminase for site-specific protein conjugation
Microbial transglutaminase for site-specific protein conjugation